Frequently Asked Questions | |
|
|
 Advanced Search Advanced Search |
| | Technology: | |
| | Method: | |
| | Effect: | |
 Basic Search Basic Search |
|
|
|
| Are Climate Catalyst particles toxic? |
|
|
|
| Are Climate Catalyst particles toxic? |
|
| A long-established principle of toxicology is that toxicity depends on dose rate (Borgert 2021). For example, eating 1 kg of table salt in one sitting could be fatal, but we all need a small amount of salt in our diet. Climate Catalyst aerosol should not be ingested or breathed in concentrated form. However, it would be difficult to collect enough Climate Catalyst aerosol particles to fill even a teaspoon without getting very close to a dispersion facility, which is not recommended. If breathed in at its normal operating concentration the effect would be like breathing in very slightly dusty air, like walking down a street. People breath in much dustier air when travelling on underground transit systems and near bonfires, barbeques and firework displays. The toxicology section (see Technical Summary) indicates that the expected concentration of climate catalyst when dispersed into a cloud or from a mountain is around a tenth [check 100th?] The safe amount allowed for a person at work breathing in the same substances continually all day. However, people and animals should not get close to a Climate Catalyst emission source for too long where the emitted particles are more concentrated in the air.Nanoparticles pose a potential hazard to fish because they can build up in their gills. However, Climate Catalyst flocculates when it falls in raindrops. This makes the particles sticky and bigger, removing the nanoparticle hazard. In moist environments such as soil Climate Catalyst particles remain gel-like and then slowly transform into clay mineral (see Technical Summary). |
|
|
|
| Are you against renewables? |
|
|
|
| Are you against renewables? |
|
| No, renewables can make sense in areas that are sufficiently windy or sunny, but that is not the case for most parts of the world. In addition, the high cost of electrical storage to smooth out renewables' intermittency has resulted in most of today's renewables deployments still needing to be backed up by fossil power. Renewables' low energy density and high resource needs means they usually have a higher overall environmental footprint than advanced nuclear. Therefore, we consider advanced nuclear to be generally more environmentally friendly than renewables, but not in all situations. Sunny, windy locations with low population densities can be better suited to renewable power. |
|
|
|
| Can action to refreeze the Arctic be delayed while more evidence is gathered? |
|
|
|
| Can action to refreeze the Arctic be delayed while more evidence is gathered? |
|
| The problem with delaying cooling to refreeze the Arctic is that the longer action is delayed, the more difficult it will become to refreeze the ice, i.e. the bigger the needed cooling influence will be to prevent warming processes tipping the climate towards a hothouse situation. Therefore, the sooner action is taken the better. |
|
|
|
| Can ocean biology be harnessed to cool the oceans? |
|
|
|
| Can ocean biology be harnessed to cool the oceans? |
|
| Yes. Increased phytoplankton growth would powerfully cool the oceans by producing more/brighter marine clouds. This is because phytoplankton emit DMS (the smell of the sea), which oxidises to nucleate clouds over the ocean. The needed very small increase in phytoplankton growth over some of the very large areas of naturally barren tropical and subtropical ocean could be achieved, for example by dispersing climate catalyst with an iron content from existing ships. That is because large areas of ocean are 'High Nutrient Low Chlorophyll' (HNLC) (Martin 1989). These HNLC areas contain all the nutrients needed for phytoplankton growth except iron. This lack of iron severely limits phytoplankton growth. Fortunately, iron is a micronutrient which means a very small amount of iron compound added to the ocean surface produces a big increase in phytoplankton growth. Other nutrients such as phosphate can be quite low in these areas, so care would need to be taken not to deplete them too much. But a well-managed aerosol fertilisation initiative could provide the urgently needed cooling at low cost, with minimal intervention and numerous additional benefits. However, ocean iron fertilisation (OIF) is another complex controversial topic that has become highly politicised. |
|
|
|
| Could Climate catalyst cause damaging effects? |
|
|
|
| Could Climate catalyst cause damaging effects? |
|
| Yes, if used improperly. If Climate Catalyst were to be emitted in polar regions with an iron content, it would fertilise growth of biofilms and algae on the ice. This would darken it, accelerating ice melt rates. Therefore, Climate Catalyst should only be dispersed near icefields without an iron content.Another unwanted effect could be changes to rainfall patterns, because brighter clouds tend to last longer before raining out. However, it's worth noting that the effects of new pollution sources on climate and rainfall patterns were rarely considered. Fortunately, some aspects of pollution had a cooling effect. But as pollution is reduced, so that cooling effect is disappearing along with any influences it might have had on rainfall patterns. Replacing that lost cooling influence with a non-toxic cooling method could bring numerous environmental benefits.Meteorologists model the effect of aerosol concentrations throughout the globe on a daily basis. Therefore, we are confident that they would be able to advise on where and when to disperse climate catalyst to produce optimal rainfall patterns. If dispersal of climate catalysts needs to be stopped for any reason its direct effects would cease within a few days or weeks when it's all rained out. The cloud cooling from increased phytoplankton emissions would cease a few weeks later. |
|
|
|
| Could Climate Catalyst enact 'cloud thinning' to refreeze the Artic during winter months? |
|
|
|
| Could Climate Catalyst enact 'cloud thinning' to refreeze the Artic during winter months? |
|
| We regard cloud thinning as a highly speculative cooling method. In theory it may be possible to make warming clouds thinner using very small particles of Climate Catalyst (perhaps 10 nm diameter). If that were possible the clouds would trap less heat, allowing more heat to escape out to space, thus slowing down ice melting rates all year round. However, a more reliable way to refreeze the Arctic would be to cool the subpolar oceans by increasing their cloud albedo. The tropical and subtropical oceans represent a major opportunity to increase cloud fraction because it is typically only around 40% there, and these waters are mainly nutrient limited only by iron. |
|
|
|
| Could Climate Catalyst refreeze the Arctic? |
|
|
|
| Could Climate Catalyst refreeze the Arctic? |
|
| Probably not just by applying it in the Arctic. Brightening Arctic clouds would help to preserve the ice for a couple of months in the summer but would increase the warming influence for the rest of the year. However, since Earth's weather systems are all interconnected there is plenty of scope for cooling other parts of the Earth that feed into the polar regions, mainly the oceans. Sufficiently cooling the oceans would help the polar ice sheets eventually to refreeze naturally. |
|
|
|
| Could climate catalyst weaken the natural methane sink by its removal of ozone? |
|
|
|
| Could climate catalyst weaken the natural methane sink by its removal of ozone? |
|
| One of the tests we want to carry out is the effect on a typical atmospheric mixture of methane and ozone of a very low concentration of climate catalyst. This would test its effect after it has spread in the air over a week or more, and the air surrounding each particle is unaffected by other distant particles. We want to ensure that single particles acting alone still have a net methane removal effect, or at least that the local removal of ozone does not weaken the existing methane sink. |
|
|
|
| Do clouds affect the climate? |
|
|
|
| Do clouds affect the climate? |
|
| Yes, in a big way. Without stratocumulus clouds, the Earth would already be an unliveable hothouse. In most parts of the world low lying clouds provide an overall cooling effect by reflecting away more heat from the sun than they trap (Ramanathan 2009). Conversely, high altitude cirrus clouds provide an overall warming influence because they trap more heat beneath them than they reflect away (Min 2010). |
|
|
|
| Do you have other ideas for cooling the climate? |
|
|
|
| Do you have other ideas for cooling the climate? |
|
| Yes, here are three further ideas we are working on: - A ship-drawn device for increasing marine cloud cover in the open ocean, by upwelling deep ocean nutrients to increase DMS emissions from boosted phytoplankton growth. - A type of floating aggregate that provides slow-release nutrients and habitat. If added in quantity to concentrated areas of the Great Pacific Garbage Patch for example, the device would provide nutrients for calcareous life such as barnacles to grow on the plastic. This would sink the plastic pieces to the seabed where they would eventually become embedded in the sediment. The main benefits would be additional cloud cooling, more fish in the sea and less plastic breaking down to microplastics that otherwise then enter the food chain. In addition, the new food source reaching the deep seabed would help to restore ecosystems there too.The aggregate stones mimic the effect of the naturally occurring pumice that is sometimes emitted in huge quantity by undersea volcanoes. Our proposed foamglass aggregate is essentially the same material (mainly silicate) with a high phosphate content and micronutrients. It's designed to last several decades before sinking. We expect a proportion to be colonised by coral and seaweed. Once the coral growth overwhelms the seaweed buoyancy the buoyant stone with its attached colony will sink quickly to the ocean bottom.The stone aggregates are made of buoyant foamglass with a mix of minerals and activated carbon grit. The activated carbon provides an anoxic environment for microbes to produce ammonium, which oxidises in the ocean water to produce nitrate. - Cloud and rain maker - a coastal based facility that produces a rising vortex of warm moist air. Bright white clouds form at the top of the vortex, that cool the land beneath them and later produce rain, especially if there are hills or mountains close inland.The Technical Summary will be provided in due course.Clive Elsworth |
|
|
|
| Does Cl atom destruction of tropospheric ozone result in less OH radicals? |
|
|
|
| Does Cl atom destruction of tropospheric ozone result in less OH radicals? |
|
| This is currently a concern that needs further research. However, the very low methane levels of the glacial periods indicate that its likely photocatalytic removal by loess dust was effective. |
|
|
|
| Does haze do much cooling? |
|
|
|
| Does haze do much cooling? |
|
| Yes, over time haze can produce as much cooling as brightening clouds (Ahlm 2017). In hot dry regions a haze may last for days before it encounters supersaturated air. Over time, humidity/water vapour builds up in the air over the ocean until it's removed by clouds that form from it, and then rain it out. |
|
|
|
| How are Climate Catalyst particles formed? |
|
|
|
| How are Climate Catalyst particles formed? |
|
| The particles can be formed easily by emitting a vapour of mixed chlorides into the air (See Technical Summary). The chlorides react rapidly with the water vapour content of the air to produce particles roughly 100 nm wide, or about 1/700 the width of a human hair. The technique of creating aerosol particles this way has been known for over a hundred years (Luo 2012). |
|
|
|
| How are today's global warming influences expected to change over time? |
|
|
|
| How are today's global warming influences expected to change over time? |
|
| Greenhouse gas emissions are expected to continue increasing, while the cooling aerosol component will continue to decrease as pollution is reduced. Thus, without intervention, global warming can be expected to accelerate further in the coming years. Interacting self-reinforcing processes will accelerate that further. |
|
|
|
| How can black carbon aerosol particles travel so far through Earth's atmosphere? |
|
|
|
| How can black carbon aerosol particles travel so far through Earth's atmosphere? |
|
| Black carbon aerosol particles absorb heat from the sun, which warms up the air around them. The warmed air produces convection currents that can carry the particles all the way up to the stratosphere (Beresnev 2017). The air in the stratosphere naturally moves towards the poles in Hadley cells with the overall effect known as Brewer Dobson circulation. That movement of air carries black carbon particles to the polar regions, where the air descends. The particles descend mainly in the winter months when there is no sun to keep them buoyant in the air (Matsui 2022). |
|
|
|
| How can climate catalyst remove black carbon aerosol particles? |
|
|
|
| How can climate catalyst remove black carbon aerosol particles? |
|
| Climate Catalyst released upwind of wildfire smoke would lighten the colour of the soot particles and make them hydrophilic by oxidation. Making the particles a lighter colour reduces their warming effect, which slows down or stops their ascent to the stratosphere. Hydrophilic particles rain out sooner. |
|
|
|
| How can removing SLCFs cool the climate if doing so releases CO2? |
|
|
|
| How can removing SLCFs cool the climate if doing so releases CO2? |
|
| 'Pound for pound' CO2 is a much less powerful greenhouse gas than all of the individual SLCFs. Therefore, the sooner SLCFs are oxidised the sooner their warming influence is curtailed. Climate catalyst removal of all SLCFs taken together immediately reduces their overall warming influence by orders of magnitude (at least hundreds of times less warming than the resulting CO2 produced). Removal of tropospheric ozone produces oxygen and OH radicals (see next FAQ), not CO2. Removal of N2O produces nitrogen and oxygen. |
|
|
|
| How can the hygroscopicity of climate catalyst particles be controlled? |
|
|
|
| How can the hygroscopicity of climate catalyst particles be controlled? |
|
| At the point of dispersal, hygroscopicity can be controlled by altering the mix of vaporous chloride precursor substances. Once in the air, particle hygroscopicity depends on droplet size. The larger the particle becomes, the less hygroscopic it becomes. Beyond a certain size, further droplet growth depends more on the relative humidity of the air it floats in. |
|
|
|
| How could such a small thing as dust cool the whole Earth? |
|
|
|
| How could such a small thing as dust cool the whole Earth? |
|
| Paleoclimate (historical) data indicate that Earth's climate is very sensitive. Earth's climate has changed a great deal in response to changes in greenhouse gas concentrations and Earth's albedo (reflectivity), albeit very slowly because the changes to these climate forcings were also slow. The albedo changes were caused mainly by ice cover changes, and quite likely cloud cover also. The glacial periods were initiated by small Earth orbital changes called the Milankovitch cycles (Buis 2020). The resulting big slow responses were caused by interacting self-reinforcing feedback mechanisms (Ripple 2023). These feedback mechanisms still operate today. |
|
|
|
| How did loess dust cool the Earth during the glacial periods? |
|
|
|
| How did loess dust cool the Earth during the glacial periods? |
|
| The increased amount of airborne loess dust (as seen in ice cores) would have produced: - a strong methane sink that kept atmospheric methane low - ocean fertilization that in each case helped to remove CO2 down to under 200 ppm, - cooling over large areas of the Earth by clouds formed: o directly from loess dust particles, o indirectly from phytoplankton emissions (Fiddes 2018).When loess dust sedimented onto the ocean surface its iron content would have fertilized extra phytoplankton growth, much as dust storms occasionally do today in areas where nutrient levels are otherwise low (Westberry 2023). That extra phytoplankton growth would have provided two of the above cooling effects. Firstly, phytoplankton emit 'the smell of the sea' which would have further increased cloud cover, further cooling large areas of ocean. Secondly, the extra phytoplankton growth would have helped to remove CO2 from the atmosphere down to its level of 200 ppm or less, although as seen in the graph above, it took thousands of years to reach that low level. |
|
|
|
| How do aerosols affect the stratospheric ozone layer? |
|
|
|
| How do aerosols affect the stratospheric ozone layer? |
|
| All aerosol particles that enter the stratosphere act there as ozone depletion catalysts. This is increasingly a problem with the black carbon aerosol component of smoke, which heats up in the air and is propelled up into the stratosphere by its own self-induced convection. Black carbon aerosol particles are strong adsorbers of ozone-depleting halogens, which they collect from the troposphere and then release into the stratosphere (Ref Solomon). This is unsurprising to chemists because black carbon particles resemble the 'activated carbon' that is often used in air filters to adsorb compounds of a similar nature from the air.However, most aerosols in the troposphere do not reach the stratosphere, so they only have an indirect effect on the stratospheric ozone layer. For example, tropospheric photocatalysts destroy methane and halogenated gases, which limits the amount of them entering the stratosphere.However, the purpose of SAI is to limit the amount of shortwave radiation entering the troposphere, which would reduce natural tropospheric methane depletion. Methane that drifts into the stratosphere readily oxidizes there, producing water as a byproduct. Water vapour is the source of the polar stratospheric clouds that today produce the ozone holes that are now observed in both the Arctic and Antarctic during each of their early springs. Thus, by inadvertently increasing water vapour in the stratosphere SAI would further damage the ozone layer (in addition to the ozone damage caused by the SAI intervention itself). Therefore, the effect of a long-term SAI intervention on natural tropospheric photocatalysts should be carefully considered before relying too heavily on SAI cooling. The same principle applies to halogenated gases and black carbon aerosol, which are also prevented from entering the stratosphere by natural tropospheric catalysts. In summary, aerosols injected into the stratosphere damage the ozone layer. They also weaken tropospheric chemistry, which in turn comes back to further damage the stratospheric ozone layer, by increasing its water vapour and black carbon aerosol content. |
|
|
|
| How do you see energy systems becoming low carbon in the future? |
|
|
|
| How do you see energy systems becoming low carbon in the future? |
|
| The most promising low carbon energy source is advanced nuclear power. Its energy density is millions of times greater than renewable energy. Modern designs promise to provide power on demand at a similar price to fossil fuels. Some of these systems can even consume recycled nuclear waste in power stations that are walkaway safe. Further, cheap process heat from advanced nuclear could replace much of the oil and gas consumed by industrial processes. That cheap, safe, low carbon heat source could also power the creation of affordable hydrogen and synthetic liquid hydrocarbon fuels suitable for aviation, for example. The CO2 needed to make synthetic fuels could be recycled from other processes, such as the burning of waste.Radiation safety is well understood by radiotherapists and nuclear engineers alike, but lack of public education and fearmongering has created public mistrust in nuclear safety. This is one of the main barriers to rapid development of advanced nuclear systems. Lack of public education on the dangers of ionizing radiation also results in people excessively tanning themselves with inadequate sunblock, risking skin cancer in later life. |
|
|
|
| How does a nitric acid coat increase the methane depletion rate? |
|
|
|
| How does a nitric acid coat increase the methane depletion rate? |
|
| In the 2014/15 ISA laboratory tests, the addition of NOx to the test chamber increased the production of Cl radicals seven-fold. That is now thought to be because NOx oxidised to then add nitrate to the particle coats. The Covid lockdown period is now thought to have contributed to an increased level of methane that built up in the atmosphere because aircraft and ships that normally emit nitric acid precursors (NOx) were grounded/moored offshore. Recent papers have shown that for naturally occurring aerosol particles a nitric acid content (HNO3) significantly boosts photoactivated production of oxidant radicals. These papers suggested that the resulting loss of airborne NOx in the air during Covid lockdowns appears to have weakened the methane sink. (Note: NOx is also produced naturally by lightning. See Technical Summary for HNO3 photocatalytic chemistry.) |
|
|
|
| How does Climate Catalyst form clouds and haze? |
|
|
|
| How does Climate Catalyst form clouds and haze? |
|
| Climate Catalyst forms clouds in the same way that naturally occurring aerosol particles do, by nucleating cloud droplets in supersaturated air. When initially dispersed it exists as bright white reflective particles that form a haze in the air. The thickness of that haze depends on humidity. |
|
|
|
| How does Climate Catalyst mimic the oxidative chemistry of Loess dust? |
|
|
|
| How does Climate Catalyst mimic the oxidative chemistry of Loess dust? |
|
| Climate catalyst utilizes the most effective catalytic ingredients of sea-salt, mineral dust aerosols and water. Its particles contain hydroxides of iron and/or titanium, which are coated by an aqueous chloride and/or nitrate coat. This combination makes the particles highly photosensitive and activated by several wavelengths of photon. Oxidant radicals are released into the air, where they oxidise SLCFs such as methane and soot particles. Reaction products then return to the aerosol particles to begin the process again in a photocatalytic cycle. This cycling around of reaction product is efficient at cleaning the air of pollution. Climate catalyst uses the same pollution removal mechanisms without the silica 'baggage' of loess dust. |
|
|
|
| How does Iron Salt Aerosol relate to Climate Catalyst? |
|
|
|
| How does Iron Salt Aerosol relate to Climate Catalyst? |
|
| Iron Salt Aerosol (ISA) was the original methane removal aerosol proposed by Franz Oeste during the early 2000s. Laboratory tests carried out in 2014/15 demonstrated its prolific chlorine radical generation. The results were published in numerous papers. (See Technical Summary.)Climate catalyst operates according to the same principles as ISA but is easier to disperse and possibly more than 10 times more photosensitive. Climate catalyst produces OH radicals in addition to Cl radicals. ISA should not be dispersed near icefields because it would fertilise growth of algae and biofilms. But climate catalyst can operate without an iron component. It can therefore be used to protect ice in polar and mountainous regions. |
|
|
|
| How effective is climate catalyst at removing SLCFs? |
|
|
|
| How effective is climate catalyst at removing SLCFs? |
|
| Several heterogenous cycles are combined by climate catalyst to enable a very small amount of chloride vapour to produce a disproportionately large amount of oxidant radicals. Depending on sun intensity and humidity, we estimate the photocatalytic cycle-driven removal of methane to enable each Cl atom to cycle around perhaps 1000 times, thus removing 1000 methane molecules before particles rain out.Unfortunately, N2O is not removed by tropospheric oxidation, and it's three times more potent than methane. However, N2O can be removed at source, making it perhaps a better candidate for efforts to reduce greenhouse gas emissions, rather than reducing methane emissions. |
|
|
|
| How is CO2 naturally removed from the atmosphere? |
|
|
|
| How is CO2 naturally removed from the atmosphere? |
|
| CO2 is mainly removed from the atmosphere by ocean absorption, photosynthesis, and rock/soil weathering. However, quite clearly these processes are not keeping up with human caused CO2 emissions. |
|
|
|
| How much must the temperature increase be limited to avoid a catastrophic slow down/halting of the AMOC? |
|
|
|
| How much must the temperature increase be limited to avoid a catastrophic slow down/halting of the AMOC? |
|
|
|
|
|
| How much will the AMOC need to slow in order to have a measurable effect on Europe’s temperature? |
|
|
|
| How much will the AMOC need to slow in order to have a measurable effect on Europe’s temperature? |
|
|
|
|
|
| How quickly can SAI be implemented? |
|
|
|
| How quickly can SAI be implemented? |
|
|
|
|
|
| If the AMOC stops, how will it take to get it started again? |
|
|
|
| If the AMOC stops, how will it take to get it started again? |
|
|
|
|
|
| In what way is Climate Catalyst safer than loess dust? |
|
|
|
| In what way is Climate Catalyst safer than loess dust? |
|
| Climate Catalyst contains a silicon based polycondensate gel, but not silica. The Climate Catalyst method produces microscopic particles that are the optimal size for cloud cooling, and they have optimal chemistry for removing powerful greenhouse gases from the air. That means, to provide the cooling needed, much less of it would need to be dispersed into the air than if loess dust were used, and in much lower concentrations. |
|
|
|
| Is Climate Catalyst sufficiently effective to be able to stabilize Earth's climate? |
|
|
|
| Is Climate Catalyst sufficiently effective to be able to stabilize Earth's climate? |
|
| Yes, it could bring temperatures down if dispersed in sufficient quantity, and in the right places at the right times. Climate Catalyst could slow down and even reverse today's accelerating changes, initiating self-reinforcing feedback cycles that multiply to provide a powerful cooling effect. In that way Climate Catalyst is fully compatible with most other (tropospheric) climate repair proposals. However, total greenhouse gas forcing eventually needs to stop increasing if cooling interventions are to keep the climate stabilised in the long-term. That means greenhouse gas emissions eventually need to be largely curtailed and/or largescale greenhouse gas removal programs will be needed. |
|
|
|
| Is CO2 a Short-Lived Climate Forcer? |
|
|
|
| Is CO2 a Short-Lived Climate Forcer? |
|
| No, CO2 normally remains in the atmosphere for thousands of years. It cannot be removed by natural oxidative processes. That is because it is already in an oxidized state. |
|
|
|
| Is it possible to make Climate Catalyst do just one thing? |
|
|
|
| Is it possible to make Climate Catalyst do just one thing? |
|
| Climate Catalyst mimics the effects of loess dust, which naturally provides many cooling effects. Some of these effects could be reduced or removed, but it would be more cost effective to mimic all the cooling effects. |
|
|
|
| Is it safe to use the ocean as a carbon sink? |
|
|
|
| Is it safe to use the ocean as a carbon sink? |
|
| The ocean has been Earth's primary carbon sink ever since it formed over 4 billion years ago. Around 40 times the amount of CO2 is dissolved in the ocean (mainly as bicarbonate) than exists in the atmosphere. Ongoingly the ocean is absorbing roughly one third of human caused CO2 emissions, which is much higher than its preindustrial absorption rate. Nonetheless, ocean lifeforms are well adapted to absorbing bicarbonate/CO2 if sufficient nutrients and habitat are available. Many trillions of tonnes of CO2 have emerged from Earth's mantle since life began and ocean life has converted that CO2 to solid sediment compounds. These are mainly carbonates such as limestone, and organic carbon such as oil, gas, shale, and kerogen that exist in huge deposits on all continents. Over time these carbon compounds, which were originally deposited in continental shelves, have been moved to the continents by tectonic and other geological processes. However, there is still disagreement on the long-term effectiveness of using the ocean as a carbon sink. In the deep open ocean (often 4 km deep or more), solid carbon compounds largely remineralize back to dissolved CO2 before they reach the seabed. That CO2 can re-emerge into the atmosphere when it reaches the sea surface. However, we see much scope for long-term carbon sequestration in shallower seas, where a larger fraction carbon compounds reaches the sediments before remineralizing. |
|
|
|
| Is smoke warming the climate as well? |
|
|
|
| Is smoke warming the climate as well? |
|
Yes. Smoke contains black carbon aerosol particles, which have a global warming potential of approximately 2000 over 20 years (Hansen et al, 2007). This is a particular problem in the Arctic, because black carbon aerosol particles emitted in the northern hemisphere tend to migrate north to form an Arctic haze that warms the ice during the winter months from October to May (Zhao 2015). In winter the haze descends, and much of it sediments in snowflakes. On the ground the water content of the snow melts and drains away, leaving a black colouring on the snow surface, accelerating the rate of snowmelt beneath it. Scientists have been surprised to find smoke particles on Arctic ice that appear to have originated from Southeast Asia.From a recent visit to Greenland, Cambridge Emeritus Professor of Ocean Physics, Peter Wadhams described the black carbon deposits on the ice as ?resembling mud'. Some of this material is likely smoke and dust particles deposited in lower layers of the ice in previous years. That ice would since have melted and drained away, leaving those particles behind from the lower layers.  Russel Glacier near Kangerlussuaq, Greenland, 1st Aug 2019courtesy of Prof Peter Wadhams. Russel Glacier near Kangerlussuaq, Greenland, 1st Aug 2019courtesy of Prof Peter Wadhams. |
|
|
|
| Is the Climate Catalyst technology patented? |
|
|
|
| Is the Climate Catalyst technology patented? |
|
| In June 2023 Climate Catalyst was patent pending. |
|
|
|
| Is there a benefit to photocatalysts producing Cl atoms rather than OH radicals? |
|
|
|
| Is there a benefit to photocatalysts producing Cl atoms rather than OH radicals? |
|
| Yes. The small percentage of methane molecules oxidized by Cl atoms happens up to 250 times faster than the equivalent reaction by OH radicals. That is because Cl atoms in dry air oxidise methane at 16 times the rate of OH radicals (Atkinson 2006). In very humid air OH radicals tend to get neutralised by water vapour or droplets, reducing their effectiveness by a factor of around 16 (Oeste 2017). These two factors of 16 combine to produce the roughly 250x factor. Mimicking natural removal of methane by Cl atoms is therefore a compelling proposition because it means potentially hundreds of times less material needs to be dispersed to remove a given amount of methane. |
|
|
|
| The AMOC has slowed considerably in the last 100 years. Has there been any measurable effect on Europe’s temperature? |
|
|
|
| The AMOC has slowed considerably in the last 100 years. Has there been any measurable effect on Europe’s temperature? |
|
|
|
|
|
| What amount of slowing down (by 2050 and 2100) must be avoided “at all costs”}? |
|
|
|
| What amount of slowing down (by 2050 and 2100) must be avoided “at all costs”}? |
|
|
|
|
|
| What are cascading tipping points? |
|
|
|
| What are cascading tipping points? |
|
| Tipping points are processes that self-reinforce and are then difficult to reverse. For example, it will likely take very cold temperatures to re-establish the Greenland ice to its pristine preindustrial form. Cascading tipping points operate in a 'domino effect', with the effect of each near-term tipping point accelerating the onset of the next one (Brovkin 2021). |
|
|
|
| What are climate catalyst particles made of? |
|
|
|
| What are climate catalyst particles made of? |
|
| Climate catalyst particles are a porous polycondensate gel of titanium hydroxide and silicic (weak) acid, with an aqueous coat of aluminium chloride (a salt). The chloride coat makes the particles hygroscopic (https://en.wikipedia.org/wiki/Aluminium_chloride), so they form an initial haze that then provides excellent cloud condensation nuclei. The particle coat may also contain nitric acid to increase their methane depletion rate (Stevenson 2022). |
|
|
|
| What are Cloud Condensation Nuclei (CCN)? |
|
|
|
| What are Cloud Condensation Nuclei (CCN)? |
|
| Cloud Condensation Nuclei are microscopic aerosol particles that initiate water vapour condensation in moist air. This is the mechanism by which clouds are formed. Cloud droplets naturally nucleate (form on aerosol particles) in air that is at the 'dew point', which is where the relative humidity is so high that dew forms on solid objects. Another way to say it is clouds form where the air is supersaturated with water vapour. Up at the level of clouds the air is by definition supersaturated. Sometimes particles nucleate at ground level, where the resulting droplets are more usually called fog or mist. |
|
|
|
| What are interacting climate feedback cycles? |
|
|
|
| What are interacting climate feedback cycles? |
|
| Climate feedback cycles tend to interact with each other, multiplying each other's effects. This makes it difficult for climate scientists to forecast how quickly today's situation will worsen. For example, ice melt causes albedo loss -> faster warming -> faster melting. A warmer Arctic weakens the jet stream, making it undulate so that some woodlands undergo longer periods without rain -> increased forest fire intensity -> more black carbon aerosol (soot). Some of that soot migrates to the Arctic -> faster melting (Backmann 2021).It should be noted that some feedback cycles operate negatively (self-correcting). For example, wildfire smoke contains traces of iron and other micronutrients that fertilise phytoplankton growth in the ocean, providing a cooling effect (Tang 2021). However, based on paleoclimate data scientists predict that if interacting feedback cycles are allowed to run their course without intervention, multi-meter sea level rise is likely this century (Hansen et al, 2023). |
|
|
|
| What are the main influences driving global warming today? |
|
|
|
| What are the main influences driving global warming today? |
|
|
|
|
|
| What are the near-term tipping points that should be avoided? |
|
|
|
| What are the near-term tipping points that should be avoided? |
|
| Both the Greenland and West Antarctic Ice Sheets are estimated to collapse at around 1.5oC (McKay 2022). The Earth is on track to reach 1.5oC average temperature increase by the 2030s, although a single year might exceed 1.5oC much sooner. The Atlantic meridional overturning circulation (AMOC) is another major tipping element in the climate system, and a future collapse would have severe impacts on the climate in the North Atlantic region. (Ditlevsen 2023). |
|
|
|
| What difference can Climate Catalyst make to each climate forcing component? |
|
|
|
| What difference can Climate Catalyst make to each climate forcing component? |
|
Here are those same IPCC figures represented as a waterfall chart: 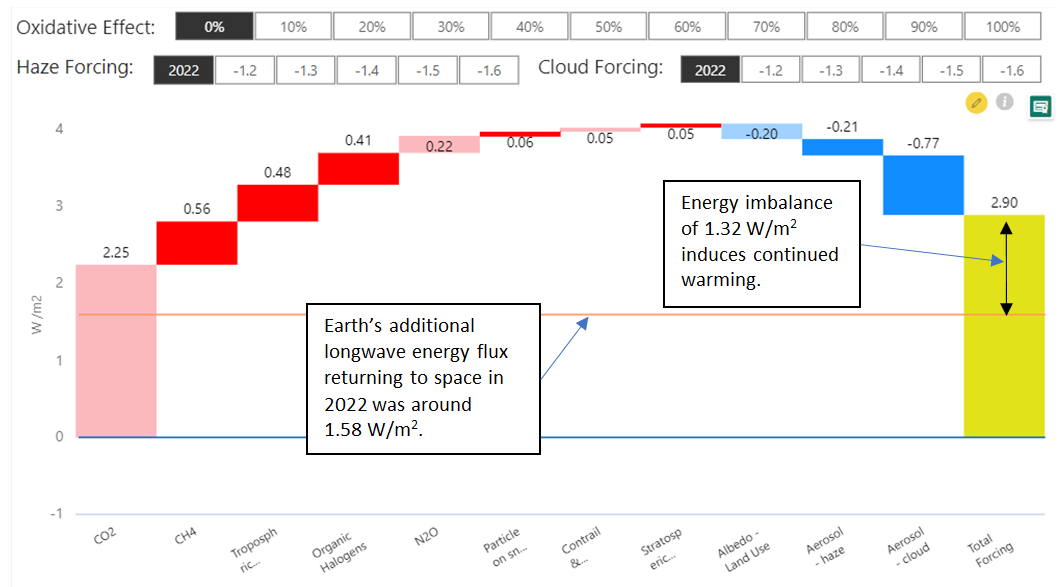 The same chart calculating 70% Climate Catalyst effectiveness shows overall radiative forcing estimated to be reduced by two thirds: The same chart calculating 70% Climate Catalyst effectiveness shows overall radiative forcing estimated to be reduced by two thirds: 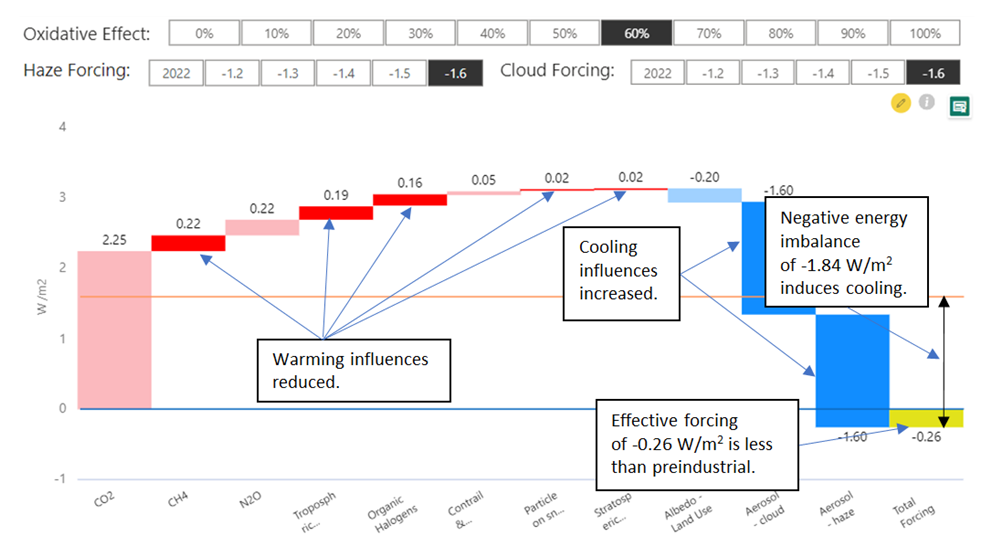 Addressable warming components are reduced by 70%. Addressable cooling is increased by 70%. Pastel colours indicate the unaffected forcings, mainly CO2. Addressable warming components are reduced by 70%. Addressable cooling is increased by 70%. Pastel colours indicate the unaffected forcings, mainly CO2. |
|
|
|
| What exactly is an aerosol? |
|
|
|
| What exactly is an aerosol? |
|
| An aerosol is a suspension of fine solid particles or liquid droplets in air (strictly, any gas). The air already naturally contains aerosol particles that are so small that they can remain airborne for weeks without sinking to the ground. This includes pollen, bacteria/viruses, and mineral dust particles. It also includes particles that form in the air from vapours emitted from sources such as human activities, vegetation, algae, and phytoplankton. For example, isoprene vapour emitted by vegetation is a major precursor of aerosol particles (Claeys 2004). Most airborne particles are removed from the air by raining or snowing out (Ardon-Dryer 2015). Only the largest particles sediment as dust. |
|
|
|
| What happens to Climate Catalyst after it gets rained out? |
|
|
|
| What happens to Climate Catalyst after it gets rained out? |
|
| Once sedimented on land or in the ocean where the pH is around 7 or above, climate catalyst particles flocculate. That means they become sticky, and they stick to other surfaces. Over weeks to months the particles poly-condense further to become a form of clay mineral, which is inert (See Technical Summary). |
|
|
|
| What happens when climate catalyst is dispersed? |
|
|
|
| What happens when climate catalyst is dispersed? |
|
| When initially dispersed, Climate Catalyst forms a white haze. The thickness of that haze depends on the relative humidity of the air it's dispersed into, and the hygroscopicity of the aerosol particles (which can be adjusted). Hygroscopic particles dispersed into humid air form a thicker more visible haze that resembles a thin fog. As with cloud formation, the particles absorb water vapour from the air to form droplets. The latent heat of condensation then releases heat that warms the haze, driving it up in the air. At higher altitude the air tends to be cooler with high relative humidity. In sufficiently moist air a cloud may form, or existing clouds will become brightened as the rising haze adds more particles to them. Those new particles 'scavenge' the water vapour evaporating from existing droplets, so existing cloud droplets get smaller. The thicker the haze or brighter the cloud, the more sunshine is reflected away, and so the greater the cooling effect. That is generally true all year round for areas outside the polar regions.A thinner haze of particles with thin aqueous coats forms from particles dispersed into dry air. These smaller droplets exhibit a lower pH because they are less diluted by water. At pH 2 or less, sunshine drives photocatalytic removal of methane and other short lived powerful greenhouse warming agents from the air. (See later FAQs). |
|
|
|
| What is a photocatalytic cycle? |
|
|
|
| What is a photocatalytic cycle? |
|
A photocatalytic cycle is a chemical reaction driven by photons that involves one or more substances 'cycling' or getting reused again and again. Removal of SLCFs involves cycling of oxidant radicals between two 'phases' (liquid and air). In this case the two phases are the liquid-covered particle phase, and the methane containing gaseous phase (the air). For example, with an aqueous ferric chloride solution coat on a loess dust particle the chlorine-atom-driven methane removal cycle involves: 1. Photons ejecting oCl atoms from the aqueous ferric chloride (FeCl3) coat of dust particles into the air. In the particle coat the ferric chloride changes to ferrous chloride (FeCl2),2. Reoxidation of ferrous chloride by oxygen to hydroxy-ferric chloride,3. oCl atoms oxidizing methane molecules in the air to form HCl,4. HCl getting absorbed back into dust particle liquid coats,5. HCl changing the hydroxy-ferric chloride back to ferric chloride and water.(The o prefix denotes the unpaired electron of a radical.)This cycling between different phases is known as heterogenous chemistry. See diagram below showing the above chemistry of an Iron Salt Aerosol (ISA) photocatalyst particle, which shows the photochemical reaction between ISA, CH4, (and other hydrocarbons) that ends in their oxidation to CO2 (Oeste et al., 2017).Heterogenous chemistry of an ISA particle 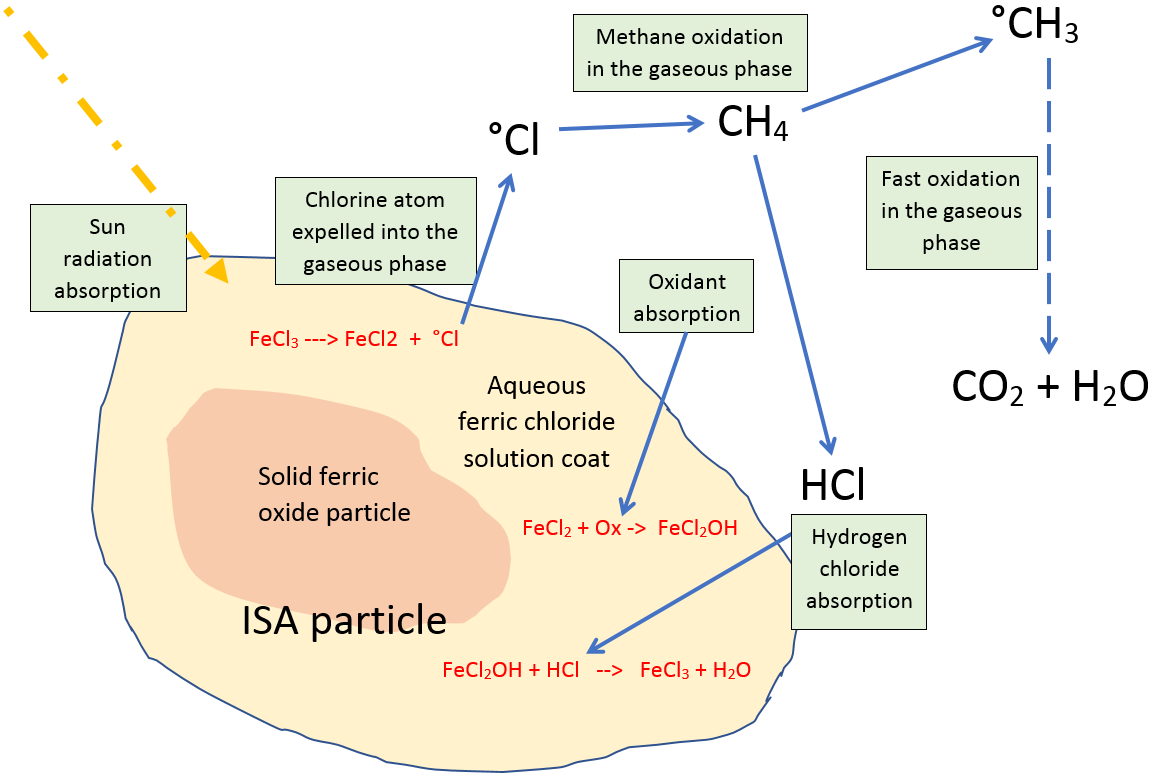 |
|
|
|
|
Loess is a very fine dust that is formed mainly by the slow grinding movement of glaciers (Smalley 2019). Glaciers always contain stones and boulders in their underside that grind the bedrock beneath. Even today, as the edges of glaciers wax and wane throughout the seasons the loess dust at their edges gets exposed and becomes airborne, driven by wind. Sometimes it travels for thousands of miles in the air (Griffin 2002).  Satellite photo of an Alaskan loess dust sediment deposit blowing over the ocean. Satellite photo of an Alaskan loess dust sediment deposit blowing over the ocean. |
|
|
|
| What is oxidation and what are oxidant radicals? |
|
|
|
| What is oxidation and what are oxidant radicals? |
|
| Oxidation is a very common class of chemical reaction (representing half of all chemistry) that occurs between oxidants and reductants. For example, methane is a chemical reductant. The reactions often involve oxygen, but not always. Oxidant 'radicals' operate in the air all the time. For example, OH radicals and single chlorine atoms (also called radicals) are sufficiently strong oxidants to oxidize individual methane molecules, thereby removing them from the air (Ming 2022). On its own, oxygen is a weaker oxidant and does not initiate methane oxidation in the air below 540oC.The OH radical is the main naturally occurring oxidant radical that removes more than 90% of the SLCFs from the air. For this reason, it has been called the 'detergent of the atmosphere' (Crutzen 1999). The naturally occurring Cl radical (single atom) removes less than 10% of the SLCFs (Hossaini 2016). |
|
|
|
| What is the best way to measure the strength of the AMOC? |
|
|
|
| What is the best way to measure the strength of the AMOC? |
|
|
|
|
|
| What is the problem with the oceans warming? |
|
|
|
| What is the problem with the oceans warming? |
|
| The warming oceans are causing numerous problems, the most noticeable of which are increased intensity of weather events such as droughts, flash floods and hurricanes. However, more serious globally damaging effects are underway. The increasingly warm ocean surface waters are increasingly buoyant because warm water is less dense than cold water. This is reducing the warm surface mixing with colder deeper waters by eddy currents. The effect is that the increasingly warmer waters flowing into the polar regions are melting some parts of the ice sheets from beneath, destabilising them. If the Atlantic is allowed to continue to warm up, the increasingly buoyant Gulfstream waters are in danger of melting the ice in the Arctic Ocean.Another effect of warmer waters flowing into the Arctic is increased evaporation from warmer waters, increasing humidity. This not only increases greenhouse warming from the increased water vapour, but cloud cover also. Unfortunately, in the polar regions for most of the year clouds trap more heat than they reflect, which means they also have an overall warming influence. These thicker clouds are therefore working to reduce winter ice regrowth rates, notably in Antarctica's 2023 winter. Already, higher Arctic temperatures are causing the Jetstream to weaken and form an undulating shape that is delivering extreme weather patterns.Another effect of warming induced density-driven loss of ocean mixing is nutrient starvation, causing reduced growth of phytoplankton in parts of the ocean. Thus, another damaging feedback mechanism is underway. Phytoplankton emissions (DMS, which is dimethyl-sulphide, or the ?smell of the sea') are the precursor of marine cloud nucleation particles, so tropical and subtropical marine clouds can be expected to decline further in the coming years. Less cloud cover means warmer waters -> less phytoplankton -> less DMS emissions -> less cloud cover and more greenhouse warming by increased humidity. This phenomenon was put forward by scientists during the 1980s as the 'Anti-CLAW' hypothesis. |
|
|
|
| What is the problem with the polar ice melting? |
|
|
|
| What is the problem with the polar ice melting? |
|
| Each summer a great deal of polar ice melts, and then more ice forms again during each winter. However, the overall trend in the Arctic is ice loss. As more ice is lost, less of the sun's heat is reflected away, so the ice is increasingly melting more rapidly, in another self-reinforcing feedback cycle. The last time the Earth experienced rapid sea level rise in a feedback cycle was around 14,000 years ago at the end of the last glacial period. Over a period of 1500 years sea level is estimated to have risen by around a meter (3 feet) every 75 years (Lambeck 2014).If the Arctic Ocean's ice all melts in the summer, much more of the sun's energy will be absorbed into it. The Greenland ice melt rate will then accelerate further, making larger and larger interventions necessary to reverse the trend. Without intervention, major sea level rise will inundate coastal settlements and arable land around the world, likely leading to famines, conflict, and the collapse of civilisation as we know it. |
|
|
|
| What natural process does Climate Catalyst mimic? |
|
|
|
| What natural process does Climate Catalyst mimic? |
|
Climate catalyst mimics the cooling effects of fine, naturally occurring mineral dust, sometimes called 'loess'. During the last million years there were numerous very cold glacial periods (often called ice ages), in which ice sheets were likely over a mile thick in parts of Canada and Europe. 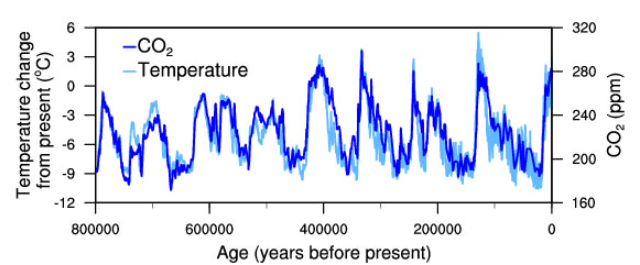 Blue arrows indicate peak glacial (coldest) periods.Some of that powerful cooling came very likely from clouds that were formed by help of airborne loess dust. That dust was sometimes more than ten times more abundant in the air than today's dust levels (Lamy et al., 2014). See graphs below. Graph (a) shows how methane levels during those cold periods were much lower than even the preindustrial level, which itself was around a third of today's methane level of around 1900 parts per billion (ppb). Graph (b) shows sea level sometimes at 120 m lower than today. Graph (c) shows the level of climate forcing (warming/cooling effect) often at minus 2-3 W/m2. For comparison, today's climate forcing is around 2.7 W/m2, i.e. around the same magnitude of influence, but in the warming direction. Blue arrows indicate peak glacial (coldest) periods.Some of that powerful cooling came very likely from clouds that were formed by help of airborne loess dust. That dust was sometimes more than ten times more abundant in the air than today's dust levels (Lamy et al., 2014). See graphs below. Graph (a) shows how methane levels during those cold periods were much lower than even the preindustrial level, which itself was around a third of today's methane level of around 1900 parts per billion (ppb). Graph (b) shows sea level sometimes at 120 m lower than today. Graph (c) shows the level of climate forcing (warming/cooling effect) often at minus 2-3 W/m2. For comparison, today's climate forcing is around 2.7 W/m2, i.e. around the same magnitude of influence, but in the warming direction. 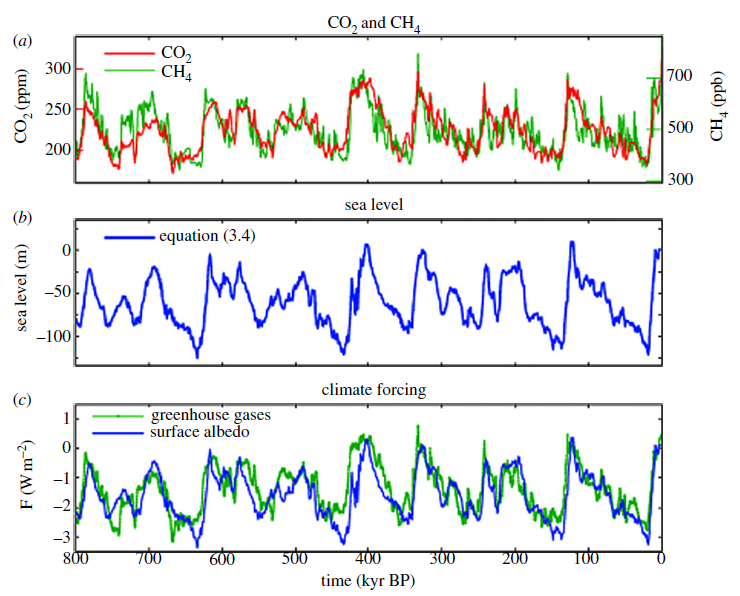 (Hansen et al, 2013)Even today, mineral dust helps to remove powerful greenhouse gases from the air (van Herpen 2023). Dust that blows over the ocean undergoes natural chemical reactions that transform small amounts of it into strong oxidative photocatalysts. Climate Catalyst mimics the most important aspects of that chemistry to similarly remove powerful greenhouse gases from the air, including methane. Methane is now (in July 2023) warming the climate almost half as much again after CO2's warming influence, so it's important to reduce methane's level in the atmosphere.Airborne dust also has a direct cooling effect by helping to form cooling clouds (McDonald, 1964). Climate Catalyst mimics that too. Since its particles are hygroscopic, they attract water vapour from moist air to form haze and to brighten clouds. By transforming water vapour into cloud droplets, the climate forcing of that airborne water turns from positive to negative. In that way, a very small mass of particles initiates a disproportionately large cooling influence. That is why we describe climate catalyst is exhibiting powerful cooling effects. (Hansen et al, 2013)Even today, mineral dust helps to remove powerful greenhouse gases from the air (van Herpen 2023). Dust that blows over the ocean undergoes natural chemical reactions that transform small amounts of it into strong oxidative photocatalysts. Climate Catalyst mimics the most important aspects of that chemistry to similarly remove powerful greenhouse gases from the air, including methane. Methane is now (in July 2023) warming the climate almost half as much again after CO2's warming influence, so it's important to reduce methane's level in the atmosphere.Airborne dust also has a direct cooling effect by helping to form cooling clouds (McDonald, 1964). Climate Catalyst mimics that too. Since its particles are hygroscopic, they attract water vapour from moist air to form haze and to brighten clouds. By transforming water vapour into cloud droplets, the climate forcing of that airborne water turns from positive to negative. In that way, a very small mass of particles initiates a disproportionately large cooling influence. That is why we describe climate catalyst is exhibiting powerful cooling effects. |
|
|
|
| What normally forms marine clouds? |
|
|
|
| What normally forms marine clouds? |
|
Sulfuric acid aerosol is the main nucleation precursor of marine clouds. The sulphuric acid is produced in several stages, beginning with phytoplankton emitting precursors that form DMS (dimethyl sulfide, 'the smell of the sea'). DMS then oxidizes in the air to form sulfur dioxide gas, which further oxidizes and hydrolyses in the air to form sulfuric acid aerosol. Comparing the two pictures below from NASA makes clear the effect of chlorophyll at producing clouds.Where there is chlorophyl there are clouds 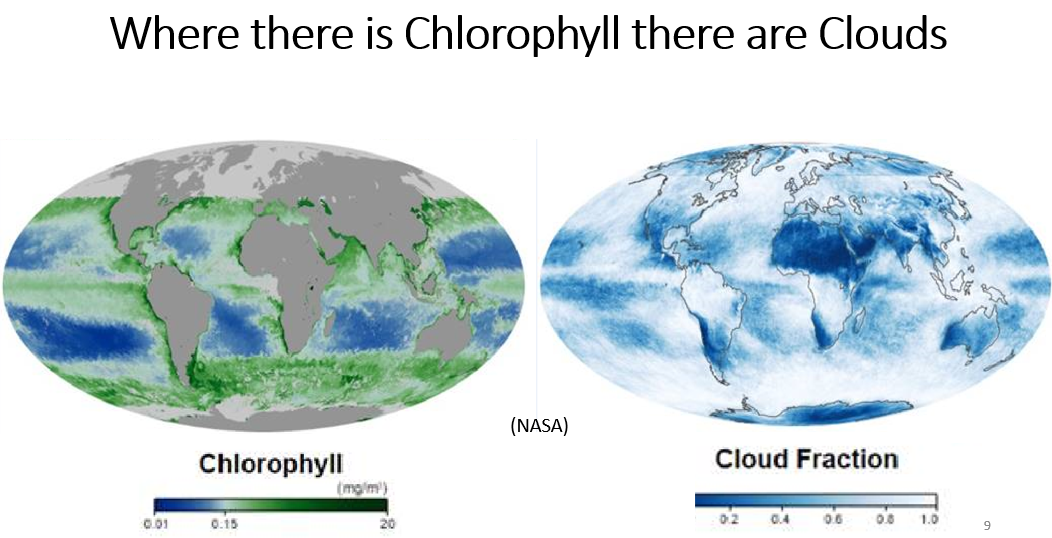 |
|
|
|
| What produces oxidant radicals in the air? |
|
|
|
| What produces oxidant radicals in the air? |
|
| The OH (hydroxyl) radical is mainly produced in the air from tropospheric ozone (Elshorbany 2010). But OH, and other oxidant radicals are additionally produced from numerous other sources. The main naturally occurring precursors of oxidant radicals are ozone, hydrogen peroxide, NOx, sea-salt, mineral acids such as sulfuric acid, and clouds. Sporadic desert dust is known to combine with these precursors to produce photosensitive compounds that accelerate oxidant radical production in the presence of sunshine (van Herpen).Such aerosol particles in the troposphere tend to be highly acidic, with the pH of their coats sometimes observed well below pH 0 (Yao 2007). When these particle coats contain photo-sensitive substances and are irradiated by sunlight they emit oxidant radicals, especially OH radicals and unpaired Cl (chlorine) atoms (Zhang 2022). For example, ferric chloride (FeCl3) produces Cl radicals in the presence of sunshine. Ferric chloride forms in the air directly over the ocean (marine boundary layer) from iron-containing mineral particles, salty sea spray, and the sulphuric acid content of the air (Vogt 1996). The main precursor of airborne sulphuric acid is phytoplankton emissions (see later).OH radicals are also produced in clouds, from the hydrogen peroxide they naturally produce (Li 2023). The presence of ferric chloride particles in clouds emits additional OH radicals during the day and night via the Fenton reaction (Deguillaume 2005). |
|
|
|
| What research is needed to make Climate Catalyst a reality? |
|
|
|
| What research is needed to make Climate Catalyst a reality? |
|
| Initially chemical laboratory tests are needed to optimise Climate Catalyst formulations for different scenarios, and to measure its toxicity. Then field tests are needed to observe its operation when added to ship flue gases, wildfire smoke, and so forth. Modern high resolution methane detection satellites should be employed to measure its efficacy. Other important work is needed to test its cloud brightening and thinning capabilities. Dispersal equipment will need to be calibrated to produce the optimal aerosols for both photocatalytic and cloud cooling operations. |
|
|
|
| What would be the effect on ocean biomass of increasing phytoplankton growth in the open ocean? |
|
|
|
| What would be the effect on ocean biomass of increasing phytoplankton growth in the open ocean? |
|
| Preindustrially, ocean wildlife populations were far higher than today. Industrial whaling and the more recent wholesale killing of large animals as bycatch in fishing operations has reduced some species by up to 99%. Whales are now famous for their role in ocean fertilization. If Climate catalyst were allowed to be dispersed with an iron component, it would very diffusely fertilize phytoplankton growth over large areas. If continued over the long-term in protected areas, some of the lost ocean ecosystems would be able to recover. That would help to restore the animal-induced mixing of nutrients near the surface, increasing phytoplankton growth further. The increased cooling cloud cover would also reduce sea surface temperatures, enabling additional nutrient mixing. We suggest this is the kind of beneficial feedback cycle that needs to be further researched, tested, and measured. |
|
|
|
| Where should climate catalyst be dispersed? |
|
|
|
| Where should climate catalyst be dispersed? |
|
| Dispersal location depends on the objective. If methane removal is the main objective climate catalyst is best dispersed in regions where the air tends to be dry. That way the methane removal rate is higher and the particles last longer before raining out. If cloud brightening is the objective, it should be dispersed in areas of marine stratiform clouds. Air turbulence mixes aerosol particles in the marine boundary layer, enabling them to reach cloud level.Climate Catalyst should be dispersed mainly in remote areas far from human habitation. The only exceptions are if it's used to keep areas at risk of wildfire moist, or to shorten the lifetime of smoke from wildfires and natural gas flares. For these purposes dispersal from unmanned aerial vehicles (UAVs or drones) would usually be most appropriate. |
|
|
|
| Which of those climate forcers is addressed by Climate Catalyst? |
|
|
|
| Which of those climate forcers is addressed by Climate Catalyst? |
|
See table below. | Climate Forcing Component | Addressed by Climate Catalyst | | CH4 (Methane) | Yes | | Halogens | Yes | | Tropospheric Ozone | Yes | | Stratospheric Water Vapour | Yes | | Light absorbing particles on snow and ice | Yes | | Aerosol - cloud | Yes | | Aerosol - radiation (haze?) | Yes | | CO2 | No | | N2O (Nitrous Oxide) | No | | Albedo - Land Use | No | | Contrails & Aviation-induced cirrus | No | |
|
|
|
| Why do Climate Catalyst particles begin their life with an acidic coat? |
|
|
|
| Why do Climate Catalyst particles begin their life with an acidic coat? |
|
| Aerosol particles naturally tend to become more acidic in the air over time. Laboratory tests carried out in 2014/15 indicated that the rate of emission of both OH and Cl radicals from photo-activated aerosol particles is highest below pH 2, and ceases much above that level (Wittmer). For maximum removal of SLCFs, climate catalyst particles should begin with their pH between around 0.5 to 2, so they can work at maximum effectiveness immediately on dispersal. Aluminium chloride keeps climate catalyst particle coats acidic until they reach supersaturated air. At that point they lose their acidity and become reflective cloud droplets instead. However, cloud droplets also naturally produce OH radicals. In this cloud mechanism smaller droplets produce more OH radicals, so brightening existing clouds is also expected to increase the atmospheric methane depletion rate, in this case by additionally producing more OH radicals in clouds. |
|
|
|
| Why do Short Lived Climate Forcers (SLCFs) have short lives in the atmosphere? |
|
|
|
| Why do Short Lived Climate Forcers (SLCFs) have short lives in the atmosphere? |
|
| Most short-lived pollutants are so-called because they are removed by the oxidative capacity of the troposphere. Naturally occurring oxidant radicals oxidize SLCFs, gradually removing them from the air. Oxidation of smoke particles turns them from hydrophobic (water repelling) to hydrophilic (water attracting), enabling them to more quickly rain or snow out. Oxidation of methane (CH4) produces CO2 and water (H2O). N2O (nitrous oxide) is removed from the atmosphere by certain types of bacteria, or it's destroyed by ultraviolet radiation or chemical reactions. |
|
|
|
| Why does brightening clouds provide a cooling influence? |
|
|
|
| Why does brightening clouds provide a cooling influence? |
|
In sub-polar regions brighter clouds reflect more sunshine away to space, so the sun doesn't warm up the air and land so much (Rosenfeld 2007). Clouds containing a higher density of smaller droplets are brighter than clouds with fewer/larger droplets. This is known as the Twomey effect (Twomey 1977). Brighter clouds form in air that has a higher-than-average concentration of CCN. See picture below of two bottles of different sized glass beads illustrating the Twomey effect.  |
|
|
|
| Why has ocean iron fertilization become so controversial? |
|
|
|
| Why has ocean iron fertilization become so controversial? |
|
| The reasons are numerous. They include the fact that knowledge is limited by the high cost of ocean research, the complexity of ocean ecosystem interactions, and fears of damaging the ocean still further, among many other reasons. However, some fears appear to be largely unjustified. For example, OIF is often cited as causing the release of neurotoxins and/or methane emissions. In this respect it doesn't help that all forms of ocean fertilisation are often lumped together. For example, the creation of intense phytoplankton blooms by releasing iron sulphate from ships is a very different proposition to very diffuse fertilisation over large areas by an iron-containing aerosol. Further, one-off fertilisation events in oligotrophic (nutrient poor) waters would have a very different effect than continuous or even sporadic fertilisation that enables a whole ecosystem to develop and adapt to a new food source. Instead of dead phytoplankton rotting in the water, a thriving ecosystem would swim up and down bringing up further nutrients from nutrient rich deep waters, just as they do elsewhere in the ocean where nutrients are plentiful.Another conundrum also leaves us puzzled. Natural fertilisation events such as dust deposition, or nutrients released from melting icebergs, or volcanic eruptions, are considered benign. But the same nutrients added artificially often seem to be considered dangerous. The ocean is the Earth's most essential climate regulation asset, and it is already overfished and polluted. Now mining companies are planning to mine it to feed the energy transition to renewables. So, it's understandable that oceanographers are reluctant to allow the ocean to be further exploited to solve human caused problems such as climate change. However, the oceans are increasingly suffering untold damage from unprecedented rapid warming. For this reason alone, the risks of inaction are beginning to outweigh the risks of enabling the ocean to enact its own natural cooling mechanisms. |
|
|
|
| Why is global warming accelerating? |
|
|
|
| Why is global warming accelerating? |
|
In addition to interacting feedback processes and natural variations such as El Niño there are two human-caused reasons. Firstly, greenhouse gas emissions are still rising. In 2023 greenhouse gas emissions became higher than ever, reaching the equivalent of 54 billion tonnes of CO2/yr (Forster 2023). Emissions will likely increase further in subsequent years as developing countries continue to grow their economies (Bhattacharya 2023). Other countries, notably China, are adding capacity to their energy mix, in part to power their planned growth of battery manufacture and other resource intensive products such as electric vehicles (Stanway 2023). Secondly, the disappearance of the cooling aerosol component of pollution is providing an additional warming influence. A case in point is the legislation (passed in Jan 2020) requiring ships to remove their sulfur dioxide emissions, which until recently cooled the ocean by forming ship tracks (Fuglestvedt 2009). See map below. 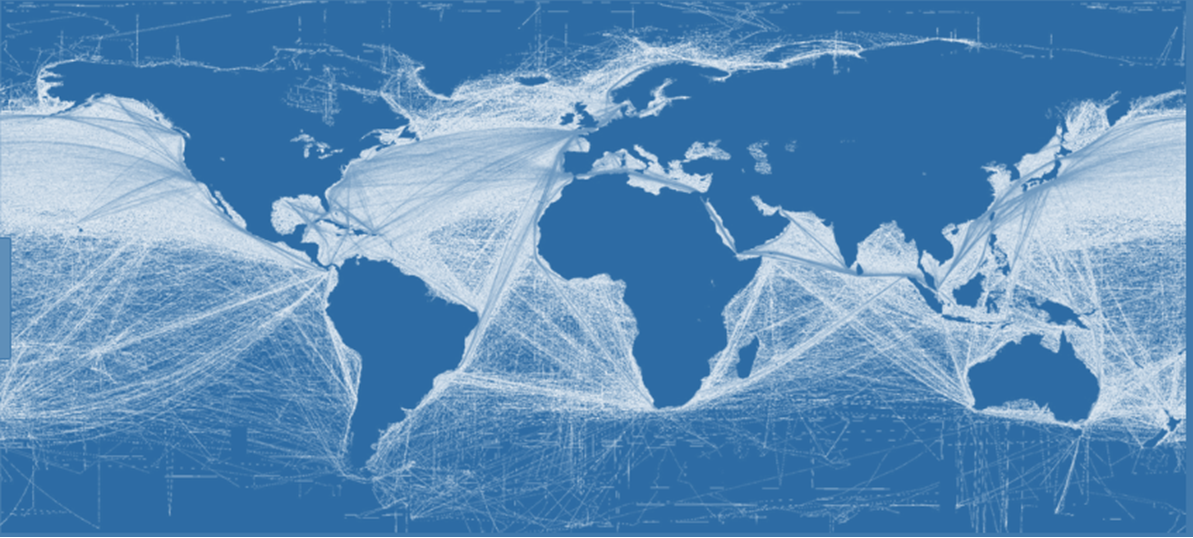 Satellite photo of superimposed ocean ship tracks before legislation reduced sulphur dioxide emissions. Satellite photo of superimposed ocean ship tracks before legislation reduced sulphur dioxide emissions. |
|
|
|
| Why not emit chlorine gas into the air to remove methane? |
|
|
|
| Why not emit chlorine gas into the air to remove methane? |
|
| Chlorine gas exists as Cl2 molecules which, in the presence of sunlight, split into Cl atoms. This would remove atmospheric methane, producing HCl as shown above. However, with no photocatalyst to recycle each Cl atom, hundreds of times the amount of chlorine gas would need to be emitted to remove a given quantity of methane than the chlorine content of climate catalyst. That would mean excessive acidification by HCl. Further, to limit regional acidification problems the chlorine gas would need to be emitted on a widespread basis. In contrast, climate catalyst particles would carry in the air removing methane over 100s or even 1000s of miles, and causing negligible acidification. |
|
|
|
| Why not just disperse loess dust into the air to cool the climate? |
|
|
|
| Why not just disperse loess dust into the air to cool the climate? |
|
| Dispersing loess dust would be much less effective than Climate Catalyst, much more expensive, and less safe. Loess dust contains silica which provides little climate benefit and is unhealthy to breathe (Dey 2017). (If you can think of loess dust as sour milk, then climate catalyst would be cheese or yoghurt - more palatable.) |
|
|
|
| Why not just pursue Net Zero emissions policies? Isn't that enough? |
|
|
|
| Why not just pursue Net Zero emissions policies? Isn't that enough? |
|
| Many climate experts say that Net Zero is now insufficient to stabilize Earth's climate (Dyke 2021). However, this is a controversial topic. Proposals to cool the climate often receive opposition from groups who prefer initiatives to be limited only to emissions reduction and carbon drawdown (UNHRC 2023).However, civilisation's appetite for energy is voracious and realistic opportunities to cut the use of fossil fuels globally are still far off. Large-scale dispatchable electricity and process heat from advanced nuclear that competes on price with fossil fuels is still decades away (Nian 2018, Goldberg 2011). Today's mining and metal ore processing capacity is nowhere near sufficient to supply a rapid energy transition to renewables (Mills 2022). For these reasons government policies to reduce emissions have so far failed. There is little reason to believe Net Zero will be much different, even though the need to reduce emissions has become urgent. Moreover, up to now no carbon drawdown technology has been implemented that can plausibly scale to remove the needed trillion tons of CO2 from the atmosphere without causing unacceptable cost and environmental damage, for example from the doubled global energy needed for Direct Air Capture systems. So, there is little reason to believe that Net Zero CO2 emissions will be reached in the next 30 years anyway.But even if Net Zero could be achieved tomorrow, scientists are now warning that slow feedbacks and climate tipping points threaten irreversible harm in the coming decades. A draft paper from veteran respected climate scientist Jim Hansen (Global Warming in the Pipeline, 2023) explains that if models based on data from Earth's paleoclimate history are used, the Earth is on track for an eventual 10oC rise. That is because even if today's greenhouse gas levels were to remain constant the oceans would continue to warm. That means tropospheric humidity would continue to increase and all ice sheets would continue to retreat, reflecting away less and less heat. However, the collapse of civilization as we know it would likely begin sooner at around 3 to 5oC, mainly due to water stress: droughts, floods, and eventually multi-meter sea-level rise. So, even if Net Zero could be successfully achieved globally unprecedented famines, mass migration, and conflict can be expected. Therefore, direct cooling methods and reduction/removal of powerful greenhouse gases are urgently needed if Earth's climate is to be stabilised. The longer the deployment of these methods is delayed the more difficult it will be to overcome the interacting self-reinforcing feedback mechanisms now in play and magnifying each other. |
|
|
|
| Why not just reduce existing methane emissions? |
|
|
|
| Why not just reduce existing methane emissions? |
|
| Today methane is leaking from hundreds of thousands of active and disused coalmines, millions of small and large oil and gas wells (Ref), oil and gas infrastructure, and sewage systems. It is also emitted by agriculture (livestock, rice paddies, slash and burn practices), landfills, wildfires, wetlands (thought to be the highest source) raw sewage discharges, millions of square kilometres of melting permafrost, and frozen methane hydrates beneath shallow Arctic seas (Shakhova 2010, Shakhova 2019). We urge policymakers to consider whether it would be more cost effective to remove methane from the atmosphere (assuming it proves safe) than to eliminate even half of these sources. The case is strengthened if the methane removal mechanism provides additional cooling effects, such as replacing the cooling component of air pollution that is increasingly disappearing. Another benefit of removing methane with climate catalyst is the removal of the black carbon aerosol content of smoke. |
|
|
|
| Why not just use Marine Cloud Brightening to cool the climate? |
|
|
|
| Why not just use Marine Cloud Brightening to cool the climate? |
|
| Marine Cloud Brightening is a promising proposal to cool the Earth and even refreeze the Arctic by brightening clouds (Salter 2018). It works by spraying microscopic seawater particles into the air from special purpose boats. Since clouds exhibit an increased oxidative effect, it should also increase the methane sink (see later). However, the technical barriers to producing the required small-sized droplets had not yet (in July 2023) been overcome. |
|
|
|
| Why not use Stratospheric Aerosol Injection (SAI) to cool the climate? |
|
|
|
| Why not use Stratospheric Aerosol Injection (SAI) to cool the climate? |
|
| SAI mimics the cooling effect of large volcanic eruptions that inject sulphur dioxide gas (SO2) into the stratosphere. SO2 oxidizes to sulfuric acid droplets, which can have a powerful cooling effect, but in that UV intense environment these particles can operate as ozone layer destroyer catalysts (Wolff 1991). Therefore, SAI is expected to further damage the ozone layer. Further, the blocking of visible light to the troposphere and an increase of damaging UV radiation (Ref Madronich) could have unknown effects on the formation of natural aerosols, the Earth's biology, and natural tropospheric photoactive air cleaning mechanisms. Without further research that could cause unexpected changes to cloud aerosol cover, damage to plants and animals, and further buildup of methane and other powerful greenhouse gases in the troposphere.Tropospheric cooling processes are more complex and difficult to understand than SAI, but it's important to recognize that they have provided most of the climate cooling throughout Earth's long history. We favour harnessing these same tropospheric cooling processes over risking the unknown consequences of mimicking long-term intensive volcanic activity.However, if cost-effective, safely scalable tropospheric cooling processes remain undeveloped, SAI should probably be used if it's the only technology available to prevent the climate situation breaching catastrophic tipping points. But as soon as tropospheric cooling mechanisms are developed and proven to be safe, we suggest they should be employed to transition from SAI, to enable a healthier troposphere and biosphere to be re-established. |
|
|
|
| Would Climate Catalyst acidify the ocean or cause acid rain? |
|
|
|
| Would Climate Catalyst acidify the ocean or cause acid rain? |
|
| No. Naturally occurring aerosol particles tend to have very acidic coats despite the amount of acid in the atmosphere over the ocean being low. This is because in dry air the airborne acid content gets highly concentrated into aerosol particle coats. The amount of climate catalyst needed to provide the required cooling would be too low to cause anything more than negligible acidification over the vast areas of remote ocean and land it would sediment onto. Indeed, we expect the opposite effect if a small iron content can be approved for ocean dispersal. The increased growth of phytoplankton would raise ocean surface pH level, countering ocean acidification. That is because the chemical assimilation of bicarbonate to organic carbon produces alkalinity, and that is what phytoplankton do when they grow (see Technical Summary.) |
|
|
|
| Would climate catalyst destroy the ozone layer? |
|
|
|
| Would climate catalyst destroy the ozone layer? |
|
| No, instead it would help it to recover, in two ways. Firstly, Climate Catalyst destroys several naturally occurring halogenated gases such as chloromethane. These gases are otherwise stable, which means they survive to drift up into the stratosphere where they break down to the halogens that destroy ozone. Secondly, climate catalyst would help to prevent black carbon aerosol drifting up into the stratosphere. Since black carbon aerosol particles are strong adsorbers of halogenated gases this would further reduce the amount of ozone destroyers reaching the stratosphere. Applying climate catalyst to the smoke of wildfires and natural gas flares would thus reduce the amount of ozone destroyers entering the stratosphere, helping the ozone layer to recover. |
|
|
|
| Would climate catalyst particles themselves reach the stratosphere? |
|
|
|
| Would climate catalyst particles themselves reach the stratosphere? |
|
| No. Unlike black carbon aerosol particles which are hydrophobic, climate catalyst particles are strongly hydrophilic (water attracting). That means they absorb water vapour from the air and rain out within a few days to weeks. Moreover, they are light-coloured and do not heat up in the sun, which means there is nothing to drive them up to the stratosphere.Moreover, all non-black, non-hydrophobic particles including climate catalyst that might drift up to high altitudes are blocked before they reach the tropopause (the boundary between troposphere and stratosphere). The air is so cold there that it effectively 'freeze dries out' aerosol particles, turning them into sinking ice crystals. Thus, the tropopause presents a barrier to entry to the stratosphere, especially for hygroscopic aerosol particles. The only circumstances in which climate catalyst particles could enter the stratosphere are unusually violent volcanic eruptions or perhaps tropical pyrocumulonimbus clouds resulting from huge wildfires. Care should therefore be taken not to apply climate catalyst in locations where these events are occurring. |
|
|
|