Climate Factors - Articles
|
|
|
|
|
|
| | # |
Name
(N/A = Not Available) |
Title |
Ref Date |
Date Added |
|---|
|
|
|
| Description |
Marine Cloud Brightening
Marine cloud brightening refers to an albedo modification technique that aims to increase the reflectivity, and possibly even the lifetimes, of certain clouds in order to reflect more sunlight back into space and partially offset some of the impacts of climate change. The most common proposal for achieving such a goal is to inject naturally occurring sea salt into cloud updrafts. But a variety of methods are being researched. |
| Contents | For example, even if marine cloud brightening could work, it could affect large scale climate and weather patterns if it were used on a large enough scale, say to achieve a level of “radiative forcing” that would be big enough to offset some of the greatest impacts of climate change. (This is because marine cloud brightening could only be implemented in limited areas, where the right kinds of clouds exist, which is perhaps only 10 percent of the planet’s surface.) There are several key risks that need to be better understood.
Still, the fact that more research is needed is true for all geoengineering techniques. So even though the scientists within our group focus most closely on stratospheric aerosol injection, we believe that MCB research is valuable. |
|
|
|
|
| Description | Summary
? Climate risks are cascading, non-linear and underestimated. Tipping points are happening
faster than forecast; some have already occurred at less than the current 1.2°C of warming.
? To provide maximum protection for the places and peoples we care about requires
returning to a climate similar to the relatively stable Holocene conditions of the last 9000
years when carbon dioxide (CO2
) levels did not exceed 280 parts per million prior to 1900.
? A “three levers” approach — “reduce, remove and repair” is required:
? Reducing emissions to zero at emergency speed;
? Removing carbon by drawdown to return atmospheric conditions to the Holocene
zone; and
? Urgent research to identify safe interventions that protect and repair vital systems
and, in the shorter term, aim to prevent warming reaching a level that triggers a
cascade of calamitous tipping points that are irreversible on human timescales.
? The harsh reality is that the first two levers alone — zero emissions and drawdown — are not
sufficient to stop the Earth system charging passing 1.5°C within the next decade (nor 2°C in
all likelihood), regardless of the emissions path.
? Even as the world moves to zero emissions, and CO2
levels start to decrease by natural
processes and by CO2 removal, albedo modification for a limited period can flatten the level
of peak warming — and perhaps help avoid existential climate impacts and extreme
damage — until the other processes fully kick in. |
|
|
|
|
Agenda
Robert C "“ Hansen 10C warming in the pipeline
John N "“ CO2 removal / warming - which follows which?
Chris V "“ UNFCCC CO2 removal document A6.4 group
Robert T "“ CCRC Tue 6th June conference - Albedo enhance, Arctic refreeze
Rebecca - Bruce Parker "“ emissions projections
Clive "“ Black carbon "“ low hanging fruit
Sev "“ Pinebank assessment, situation and solutions
John N - Changing the narrative to albedo enhancement / SRM
Chat
21:15:23 From Rebecca Bishop - Gadigal lands : What convivial and productive group dynamics we have this morning :-)
21:20:21 From Mannajo Greene : Reacted to |  (Click HERE to view the transcrript) (Click HERE to view the transcrript) |
|
|
|
|
Agenda
Franz "“ UV loss from SAI, affecting tropospheric oxidative capacity
Robert T "“ Albedo effects of Iron Salt Aerosol
Chris V "“ 60-70 scientists call for SRM research
John M "“ template for outreach
20:06:27 From Shaun Fitzgerald : Letter |  (Click HERE to view the transcrript) (Click HERE to view the transcrript) |
|
|
|
|
Agenda
Bru - feedback from Geneva keynote speech
Bru – New Legislation / research on ocean restoration
Chris – Ocean CDR reporting and verification – useful for permitting, ultimately payment. Good talk – chat link
Clive / Stephen – questions on typhoon moderation - MCB calculations
John N – Question: Who in authority is in favour of SRM – Albedo enhancement: Research? / Deployment?
Chat:
21:03:12 From Bru Pearce : The US congress has just now had new legislation introduced to put the federal government on board with us with the new “Ocean Restoration Research and Development Act 2022â€. https://www.conservamerica.org/latest-news/conservamerica-welcomes-introduction-of-ocean-restoration-legislation
21:04:18 From Chris Vivian : Marine Carbon Dioxide Removal: Essential Science and Problem Solving for Measurement, Reporting, and Verification Workshop - https://www.us-ocb.org/marine-co2-removal-workshop/
21:09:26 From Chris Vivian : Excellent talk ‘MRV for Ocean-Based CDR Methods’ by Dr Jessica Cross, NOAA at https://youtu.be/VXhCa6jKsHQ
21:52:25 From Robert Tulip : Albedo has fallen by half a watt per square metre in the last twenty years. https://agupubs.onlinelibrary.wiley.com/doi/full/10.1029/2021GL094888
22:04:23 From Bru Pearce : https://www.energy.gov/fecm/articles/us-department-energy-announces-30-million-remove-carbon-dioxide-air-and-oceans-and
22:06:28 From Bru Pearce : https://envisionationorg.sharepoint.com/:b:/r/sites/restorationplan/Shared%20Documents/Released/Biosphere%20Restoration%20Plan%202022.pdf?csf=1&web=1&e=8vqzyZ
22:09:00 From Dr Brian von Herzen : SharePoint not accessible
22:13:31 From Chris Vivian : https://theconversation.com/climate-scientists-concept-of-net-zero-is-a-dangerous-trap-157368
22:13:49 From Dr Brian von Herzen : thank you Robert, earthshine paper is very helpful
22:16:17 From John Nissen : Earthshine paper may be biased if looking at reflection from moon, as equator bias.
22:16:45 From Dr Brian von Herzen : Ceres data supports it
22:17:38 From Dr Brian von Herzen : Ceres shows 1w/m² decline over the same period
22:17:59 From Dr Brian von Herzen : satellite observation
22:18:43 From John Nissen : @Brian interesting!
22:19:05 From Dr Brian von Herzen : ðŸ‘
22:19:22 From John Nissen : Have they measured IR change?
22:31:51 From Bru Pearce : Good night all I have run out of steam. email me directly if you want a copy of my paper. |  (Click HERE to view the transcrript) (Click HERE to view the transcrript) |
|
|
|
|
|
| | # |
Name
(N/A = Not Available) |
Title |
Ref Date |
Date Added |
|---|
|
|
|
| Description | This is the second article in a two-part series. Read the first part here.
Climate model scenarios similar to current policies project 2°C of warming before 2050; if James Hansen is right (see Part 1) and warming sharply accelerates, it could be a decade sooner. These outcomes will be driven by the high energy imbalance, continuing high emissions, the accelerating accumulation of heat in the oceans, and decreases in short-term aerosol cooling. |
| Contents | Several years ago a group of eminent scientists proposed a “carbon law”, which said that keeping warming to 2°C required emissions to be halved every decade from 2020 onwards, including a halving between 2020 and 2030, plus some carbon drawdown. Instead, the level of greenhouse gases and coal use both hit record highs in 2023. And the largest national fossil fuel producers plan to keep on expanding production As a result, current government plans worldwide will likely result in emissions in 2050 almost as high as they are today, according to the UN Environment Programme’s 2023 Production Gap report.
Other analyses are broadly consistent:
The International Energy Agency finds that stated national policies will result in oil and gas production in 2050 as high as 2020; with coal halved.
The OECD finds that a world economy four times larger than today is projected to need 80% more energy in 2050; and without new policy action and the global energy mix in 2050 will not differ significantly from today.
The intentions of the world’s five largest fossil fuel producers are clear — and civilisation-threatening — as reported by the UN:
In China, oil production is projected to be flat to 2050, but gas will increase more than 60 percent from 2020 to 2050, while coal use will remain high till 2030 then decline sharply.
In the United States, oil production will grow and then remain at record levels to 2050, and gas is projected to continuously and significantly increase to 2050; whilst coal will drop by half.
Projections for Russia are available only to 2035, with coal and gas production projected to increase significantly, while oil remains flat.
In Saudi Arabia, oil production is projected to grow by 26 to 47 percent by 2050, with gas up 40 percent between 2019 and 2050. Together they make up half of the Saudi economy.
And in Australia, one of the world’s top two liquified natural gas and coal exporters, gas production is projected to stay above the current level for the next 15 years, with coal remaining high over the same period, above 450 million metric tons annually.
We are heading towards 3–4°C.
This outlook suggests Earth is heading towards 3°C of warming and perhaps a good deal more, because current climate models which project warming of around 2.7°C do not adequately account for all the system-level reinforcing feedbacks.
In 2021, the pre-eminent UK international affairs think-tank Chatham House said a “plausible worst-case scenario” is 3.5°C or more, which could be an underestimate if tipping points are reached sooner than the orthodox science suggests. This now seems to be the reality.
A clear majority of scientists expected warming of more than 3°C, and 82% expected to see catastrophic impacts of climate change in their lifetime, according to a 2021 survey by the journal Nature.
Questions about the size of the aerosol forcing, and the related issue of how sensitive the climate is to changes in greenhouse gases, remain an issue of scientific contention.
New climate history research published in December 2023, based on a study of the last 66 million years, concluded that global temperature may be more sensitive to CO2 levels than current models estimate. It showed that the last time CO2 levels were as high as today was around 14 million year ago, which is longer than previous estimates, and that climate sensitivity — the amount of warming resulting from a doubling of atmospheric CO2 — may be between between 5°C and 8°C, compared to the IPCC orthodoxy of 1.5–4.5°C.
The level of greenhouse gases is currently around 560 parts per million, double the pre-industrial level. Some of those gases such as methane are short-lived so this level of forcing is not written in stone, but nevertheless if Hansen et al. are right that a doubling may lead to around 4–5°C of warming, then another 30 years of high emissions means humans will have created an increasingly unliveable planet.
Has the impact of aerosols been widely understood? In what the New York Times described as “an eye-opening Nature commentary”, Geeta Persad and her colleagues wrote in late 2022 that “overall, vast emissions of aerosols since the start of the industrial age have had a profound cooling effect” and that without them “the global warming we see today would be 30 to 50 percent greater”, warning that “the impacts of aerosols on climate risk are often ignored”.
In 2018, a group of eminent scientists explored the potential — once warming had exceeded the 1.5–2°C range — for self-reinforcing positive feedbacks in major elements of the climate system to push passed a planetary threshold that would prevent temperature stabilisation, and drive the system to a “Hothouse Earth”. They warned that “we are in a climate emergency… this is an existential threat to civilisation”.
The 2023 State of the Climate Report: Entering uncharted territory warned of: “potential collapse of natural and socioeconomic systems in such a world [of 2.6°C warming] where we will face unbearable heat, frequent extreme weather events, food and fresh water shortages, rising seas, more emerging diseases, and increased social unrest and geopolitical conflict.”
Whatever the words, the understanding is widely shared that contemporary nations and societies, and likely the global social system, are heading towards collapse. “If we carry on the way we are going now, I can’t see this civilisation lasting to the end of this century”, says Professor Tim Lenton. The US Defence Secretary Lloyd Austin III calls the risks “existential”.
Opening the Innovation Zero Congress in London in May 2023, Potsdam Institute Director Prof. Johan Rockstrom described the path we are on:
“2.5°C global mean surface temperature rise is a disaster. It’s something that humanity has absolutely no evidence that we can cope with… [There] would be a 10-metre sea-level rise. There would be a collapse of all the big biomes on planet Earth – the rainforest, many of the temperate forests – abrupt thawing of permafrost, we will have complete collapse of marine biology… Over one-third of the planet around the equatorial regions will be uninhabitable because you will pass the threshold of health, which is around 30°C. It’s only in some parts of the Sahara Desert today that has that kind of average temperature.”
Chatham House’s Climate Risk Assessment 2021 concludes that by 2050 global food demand would be 50% higher, but crop yields may drop by 30%. As desertification spreads across the dry sub-tropics, and one-third of the planet experiences unprecedented heat, it is not difficult to see why they concluded that cascading climate impacts will “drive political instability and greater national insecurity, and fuel regional and international conflict”.
What is worse is the setback to climate action posed by current conflicts and military posturing in Europe, the Middle East and east Asia, which are huge political distractions from dealing with the greatest threat to humanity, and all of which have the potential to spread more widely.
To maintain military flexibility, the US insisted in 1997 that direct military carbon emissions be excluded from international carbon accounting. Those emissions, around 5 percent of the total global, are far less than the indirect emissions from conflict, as recent estimates here and here indicate.
Projections show that by 2100 the expansion of the Sahara due to desertification will embrace Israel/Palestine, as well as spreading across the Mediterranean into Spain, Italy, Greece and Turkey (see map).
The Australian Prime Minister has finally spoken out about the escalating climate threat whilst inspecting damage from the recent Queensland floods: “All of this is a reminder that the science told us that climate change would mean there would be more extreme weather events and they would be more intense. And unfortunately, we are seeing that play out with the number of events that we’re having to deal with right around Australia”.
Just so, except that in common with leaders globally, the Australian government continues to have its head stuck in the sand about the real risks climate change now represents. It refuses to release an intelligence assessment of climate-security risks, and has fumbled a domestic climate risk assessment.
As a result, the community remains ill-informed and unprepared for what is coming. |
|
|
|
|
| Author | David Spratt and Ian Dunlop |
| Description | For climate change, 2023 was an “unprecedented” year, “absolutely gobsmackingly bananas” and “scary” and “frightening”. And that was what climate scientists said! The UN Secretary General called it the year in which humanity crossed into a new climate era — an age of “global boiling”.
Climate disruption shocked climate scientists in 2023. “Surprising. Astounding. Staggering. Unnerving. Bewildering. Flabbergasting. Disquieting. Gobsmacking. Shocking. Mind boggling,” said Prof. Ed Hawkins when September 2023 exceeded the previous September record by a huge 0.5°C.
The decline in Antarctic sea-ice extent was much greater than model projections, leading the National Snow and Ice Data Centre’s Walt Meier to exclaim: “It’s so far outside anything we’ve seen, it’s almost mind- blowing.”
Many records were set for new climate extremes — record heat, rainfall and floods — with some of it driven by the destabilisation of the polar jet stream. “We are hitting record breaking extremes much sooner than I expected. That’s frightening, scary, and concerning, and it really suggests that we’re not as aware of what’s coming as we thought we were,” said Sarah Perkins-Kirkpatrick of the University of NSW. |
| Contents | With devastating extreme heat and storms and floods, 2023 was the first year 1.5°C warmer than the 1850-1900 baseline, and both Antarctic sea-ice loss and record northern hemisphere sea-surface temperatures were way beyond the ranges projected by climate models.
Datasets of global temperatures vary a little depending on method, but two of the most significant are Berkeley Earth which put 2023 at 1.54°C above the pre-industrial (1850-1900) level, and Copernicus/ECMWF at 1.48°C.
Berkeley said that “a single year exceeding 1.5°C is a stark warning sign of how close the overall climate system has come to exceeding this Paris Agreement goal. With greenhouse gas emissions continuing to set record highs, it is likely that climate will regularly exceed 1.5°C in the next decade.”
2023 was notable for:
Global average warming hitting the 1.5°C mark, and new monthly records for global temperature every month from June to December. The October to December period was 1.74°C.
New national record high annual averages for an estimated 77 countries.
The first year that global average ocean surface temperatures exceeded 1°C, with once-in-a-century levels of warmth in the North Atlantic.
Two days in November when global average temperature, for the first time ever, reached 2°C above the pre-industrial levels.
Catastrophic flooding from Greece to Beijing to Vermont, and earlier in the year major flooding in New Zealand associated with a rain bomb and then cyclone Gabrielle.
Severe wildfires in Europe, Russia, Maui and North America; fires in Canada burned 18.5 million hectares of land.
The 2023 extremes were a shock. Prof. Katharine Hayhoe told the Guardian that: “We have strongly suspected for a while that our projections are underestimating extremes, a suspicion that recent extremes have proven likely to be true… We are truly in uncharted territory in terms of the history of human civilisation on this planet.”
Explanations for 2023 are incomplete, but warming is accelerating and 2024 is likely to be hotter
What happened in 2023 was not what scientists’ models anticipated at the beginning of the year and fell well outside the confidence intervals of any of the estimates. Carbon Brief says that “while there are a number of factors that researchers have proposed to explain 2023’s exceptional warmth, scientists still lack a clear explanation for why global temperatures were so unexpectedly high… researchers are just starting to disentangle the causes of the unexpected extreme global heat the world experienced in 2023”.
One person who has a clear view is the former NASA climate chief James Hansen who says that “the 1.5 degree limit is deader than a doornail” and warns that warming will accelerate to 1.7°C by 2030 and “2°C will be reached by the late 2030s”. |
|
|
|
|
| Description | Envisionation is a unique thinktank and consulting group made up of an international network of critical system thinkers, climate scientists, economists, business entrepreneurs and journalists, working to understand the functioning of the Whole Earth System and the full extent of the human impact on it.
We draw the individual components together to provide an overarching view of the whole, providing our clients with essential information to navigate the future, enabling them to identify threats, contingent liabilities and the vast opportunities that restoring Earth’s biosphere present. |
| Contents | Because climate change and environmental degradation impact everything
Children whose future has and is being compromised
Climate scientists, who recognise the shortcomings of the inadequacy of proposed responses
Campaigners looking for solutions rather than just screaming, “Do something!”
Politicians and leaders, who need positive plans and messages to encourage voters
Businesses concerned about their fiduciary responsibilities to their shareholders, employees, and customers
Bankers, insurers and hedge funds that need to take action to preserve the value of their portfolios
Hydrocarbon companies that grasp the need to transition |
|
|
|
|
| Description | Yale Climate Connections is a news service that aims to help you understand the reality of climate change and what you can do about it. Through our website, YouTube channel, and national radio program, which airs each day on hundreds of stations, we reach millions of people like you each year.
|
| Contents | We are staffed by professional journalists, meteorologists, and radio producers. We’re independent and nonpartisan. Yale Climate Connections is an initiative of the Yale Center for Environmental Communication, directed by Dr. Anthony Leiserowitz of the Yale School of the Environment at Yale University.
Most content is published under Creative Commons: Attribution-Noncommercial-No Derivative Works. Please visit our Use & Privacy Policy page for additional information.
Yale Climate Connections is grateful for the generous financial support of the Grantham Foundation for the Protection of the Environment and of individual Yale University alumni. We also thank the CO2 Foundation for its support of our Spanish-language articles. Yale Climate Connections is solely responsible for all content. |
|
|
|
|
| Description | Global heating of the Earth system is unequivocal. However, detecting an acceleration of Earth heating has remained elusive to date, despite suggestive evidence of a potential increase in heating rates. In this study, we demonstrate that since 1960, the warming of the world ocean has accelerated at a relatively consistent pace of 0.15 ± 0.05 (W/m2)/decade, while the land, cryosphere, and atmosphere have exhibited an accelerated pace of 0.013 ± 0.003 (W/m2)/decade. This has led to a substantial increase in ocean warming, with a magnitude of 0.91 ± 0.80 W/m2 between the decades 1960-1970 and 2010-2020, which overlies substantial decadal-scale variability in ocean warming of up to 0.6 W/m2. Our findings withstand a wide range of sensitivity analyses and are consistent across different observation-based datasets. The long-term acceleration of Earth warming aligns qualitatively with the rise in CO2 concentrations and the decline in aerosol concentration during the same period, but further investigations are necessary to properly attribute these changes. |
| Contents | In the past 150 years, Earth's climate has been warming at a rate that is unprecedented in at least the last 2000 years1. This human-caused warming has caused widespread adverse impacts and related losses and damages to nature and people, which will continue in the future as global climate continues to warm2. Detecting changes in the rate of warming is crucial for informed decision-making in international climate negotiations, with the aim of limiting global warming to specific levels. However, it remains a significant challenge to detect such changes due to the substantial internal variability of the climate system on a decadal scale (e.g., ref. 3). In this paper, we address this challenge by examining the global heat accumulation rate across the entire climate system, including the ocean, atmosphere, cryosphere, and land. By focusing on this integrated view, rather than solely relying on changes in global mean surface temperature, we can mitigate the impact of variability and gain a more comprehensive understanding4,5. Global heat accumulation in the climate system, resulting from the current positive Earth's Energy Imbalance (EEI) at the top of the atmosphere, is primarily dominated by changes in Global Ocean Heat Content (GOHC)4. GOHC changes account for approximately 90% of the total heat increase in the past fifty years, while land heating, ice melting, and atmospheric warming contribute around 5%, 3%, and 1% respectively6,7,8. Several studies have indicated an increase in the global heat accumulation rate in recent decades, with values rising from 0.50 [0.32 to 0.69] W/m2 during the period 1971-2006 to 0.79 [0.52 to 1.06] W/m2 (90% confidence interval) for the period 2006-2018 (ref.4,6,7,8,9,10,11,12,13,14,15,16,17,18,19,20,21,22 and Fig. 1). Some studies have even suggested a potential doubling of EEI in the last decade compared to the previous one6,17. Figure 1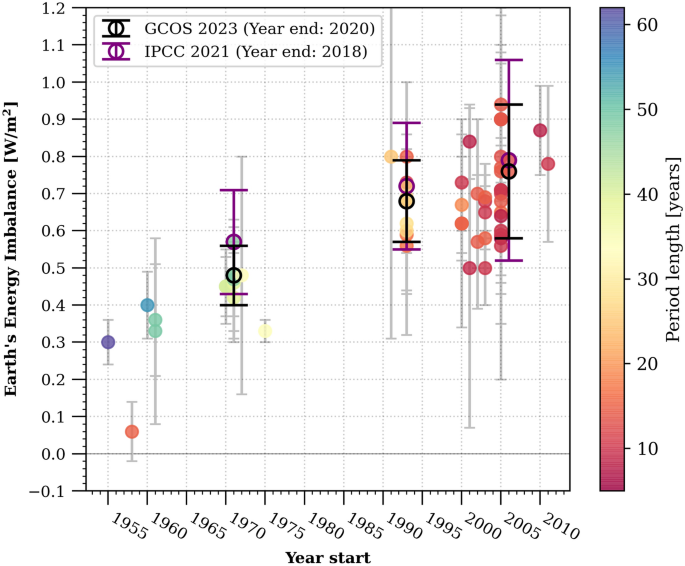 |
|
|
|
|
|
| | # |
Name
(N/A = Not Available) |
Title |
Ref Date |
Date Added |
|---|
|
|
|
| Description | This is the second article in a two-part series. Read the first part here.
Climate model scenarios similar to current policies project 2°C of warming before 2050; if James Hansen is right (see Part 1) and warming sharply accelerates, it could be a decade sooner. These outcomes will be driven by the high energy imbalance, continuing high emissions, the accelerating accumulation of heat in the oceans, and decreases in short-term aerosol cooling. |
| Contents | Several years ago a group of eminent scientists proposed a “carbon law”, which said that keeping warming to 2°C required emissions to be halved every decade from 2020 onwards, including a halving between 2020 and 2030, plus some carbon drawdown. Instead, the level of greenhouse gases and coal use both hit record highs in 2023. And the largest national fossil fuel producers plan to keep on expanding production As a result, current government plans worldwide will likely result in emissions in 2050 almost as high as they are today, according to the UN Environment Programme’s 2023 Production Gap report.
Other analyses are broadly consistent:
The International Energy Agency finds that stated national policies will result in oil and gas production in 2050 as high as 2020; with coal halved.
The OECD finds that a world economy four times larger than today is projected to need 80% more energy in 2050; and without new policy action and the global energy mix in 2050 will not differ significantly from today.
The intentions of the world’s five largest fossil fuel producers are clear — and civilisation-threatening — as reported by the UN:
In China, oil production is projected to be flat to 2050, but gas will increase more than 60 percent from 2020 to 2050, while coal use will remain high till 2030 then decline sharply.
In the United States, oil production will grow and then remain at record levels to 2050, and gas is projected to continuously and significantly increase to 2050; whilst coal will drop by half.
Projections for Russia are available only to 2035, with coal and gas production projected to increase significantly, while oil remains flat.
In Saudi Arabia, oil production is projected to grow by 26 to 47 percent by 2050, with gas up 40 percent between 2019 and 2050. Together they make up half of the Saudi economy.
And in Australia, one of the world’s top two liquified natural gas and coal exporters, gas production is projected to stay above the current level for the next 15 years, with coal remaining high over the same period, above 450 million metric tons annually.
We are heading towards 3–4°C.
This outlook suggests Earth is heading towards 3°C of warming and perhaps a good deal more, because current climate models which project warming of around 2.7°C do not adequately account for all the system-level reinforcing feedbacks.
In 2021, the pre-eminent UK international affairs think-tank Chatham House said a “plausible worst-case scenario” is 3.5°C or more, which could be an underestimate if tipping points are reached sooner than the orthodox science suggests. This now seems to be the reality.
A clear majority of scientists expected warming of more than 3°C, and 82% expected to see catastrophic impacts of climate change in their lifetime, according to a 2021 survey by the journal Nature.
Questions about the size of the aerosol forcing, and the related issue of how sensitive the climate is to changes in greenhouse gases, remain an issue of scientific contention.
New climate history research published in December 2023, based on a study of the last 66 million years, concluded that global temperature may be more sensitive to CO2 levels than current models estimate. It showed that the last time CO2 levels were as high as today was around 14 million year ago, which is longer than previous estimates, and that climate sensitivity — the amount of warming resulting from a doubling of atmospheric CO2 — may be between between 5°C and 8°C, compared to the IPCC orthodoxy of 1.5–4.5°C.
The level of greenhouse gases is currently around 560 parts per million, double the pre-industrial level. Some of those gases such as methane are short-lived so this level of forcing is not written in stone, but nevertheless if Hansen et al. are right that a doubling may lead to around 4–5°C of warming, then another 30 years of high emissions means humans will have created an increasingly unliveable planet.
Has the impact of aerosols been widely understood? In what the New York Times described as “an eye-opening Nature commentary”, Geeta Persad and her colleagues wrote in late 2022 that “overall, vast emissions of aerosols since the start of the industrial age have had a profound cooling effect” and that without them “the global warming we see today would be 30 to 50 percent greater”, warning that “the impacts of aerosols on climate risk are often ignored”.
In 2018, a group of eminent scientists explored the potential — once warming had exceeded the 1.5–2°C range — for self-reinforcing positive feedbacks in major elements of the climate system to push passed a planetary threshold that would prevent temperature stabilisation, and drive the system to a “Hothouse Earth”. They warned that “we are in a climate emergency… this is an existential threat to civilisation”.
The 2023 State of the Climate Report: Entering uncharted territory warned of: “potential collapse of natural and socioeconomic systems in such a world [of 2.6°C warming] where we will face unbearable heat, frequent extreme weather events, food and fresh water shortages, rising seas, more emerging diseases, and increased social unrest and geopolitical conflict.”
Whatever the words, the understanding is widely shared that contemporary nations and societies, and likely the global social system, are heading towards collapse. “If we carry on the way we are going now, I can’t see this civilisation lasting to the end of this century”, says Professor Tim Lenton. The US Defence Secretary Lloyd Austin III calls the risks “existential”.
Opening the Innovation Zero Congress in London in May 2023, Potsdam Institute Director Prof. Johan Rockstrom described the path we are on:
“2.5°C global mean surface temperature rise is a disaster. It’s something that humanity has absolutely no evidence that we can cope with… [There] would be a 10-metre sea-level rise. There would be a collapse of all the big biomes on planet Earth – the rainforest, many of the temperate forests – abrupt thawing of permafrost, we will have complete collapse of marine biology… Over one-third of the planet around the equatorial regions will be uninhabitable because you will pass the threshold of health, which is around 30°C. It’s only in some parts of the Sahara Desert today that has that kind of average temperature.”
Chatham House’s Climate Risk Assessment 2021 concludes that by 2050 global food demand would be 50% higher, but crop yields may drop by 30%. As desertification spreads across the dry sub-tropics, and one-third of the planet experiences unprecedented heat, it is not difficult to see why they concluded that cascading climate impacts will “drive political instability and greater national insecurity, and fuel regional and international conflict”.
What is worse is the setback to climate action posed by current conflicts and military posturing in Europe, the Middle East and east Asia, which are huge political distractions from dealing with the greatest threat to humanity, and all of which have the potential to spread more widely.
To maintain military flexibility, the US insisted in 1997 that direct military carbon emissions be excluded from international carbon accounting. Those emissions, around 5 percent of the total global, are far less than the indirect emissions from conflict, as recent estimates here and here indicate.
Projections show that by 2100 the expansion of the Sahara due to desertification will embrace Israel/Palestine, as well as spreading across the Mediterranean into Spain, Italy, Greece and Turkey (see map).
The Australian Prime Minister has finally spoken out about the escalating climate threat whilst inspecting damage from the recent Queensland floods: “All of this is a reminder that the science told us that climate change would mean there would be more extreme weather events and they would be more intense. And unfortunately, we are seeing that play out with the number of events that we’re having to deal with right around Australia”.
Just so, except that in common with leaders globally, the Australian government continues to have its head stuck in the sand about the real risks climate change now represents. It refuses to release an intelligence assessment of climate-security risks, and has fumbled a domestic climate risk assessment.
As a result, the community remains ill-informed and unprepared for what is coming. |
|
|
|
|
| Description | Global heating of the Earth system is unequivocal. However, detecting an acceleration of Earth heating has remained elusive to date, despite suggestive evidence of a potential increase in heating rates. In this study, we demonstrate that since 1960, the warming of the world ocean has accelerated at a relatively consistent pace of 0.15 ± 0.05 (W/m2)/decade, while the land, cryosphere, and atmosphere have exhibited an accelerated pace of 0.013 ± 0.003 (W/m2)/decade. This has led to a substantial increase in ocean warming, with a magnitude of 0.91 ± 0.80 W/m2 between the decades 1960-1970 and 2010-2020, which overlies substantial decadal-scale variability in ocean warming of up to 0.6 W/m2. Our findings withstand a wide range of sensitivity analyses and are consistent across different observation-based datasets. The long-term acceleration of Earth warming aligns qualitatively with the rise in CO2 concentrations and the decline in aerosol concentration during the same period, but further investigations are necessary to properly attribute these changes. |
| Contents | In the past 150 years, Earth's climate has been warming at a rate that is unprecedented in at least the last 2000 years1. This human-caused warming has caused widespread adverse impacts and related losses and damages to nature and people, which will continue in the future as global climate continues to warm2. Detecting changes in the rate of warming is crucial for informed decision-making in international climate negotiations, with the aim of limiting global warming to specific levels. However, it remains a significant challenge to detect such changes due to the substantial internal variability of the climate system on a decadal scale (e.g., ref. 3). In this paper, we address this challenge by examining the global heat accumulation rate across the entire climate system, including the ocean, atmosphere, cryosphere, and land. By focusing on this integrated view, rather than solely relying on changes in global mean surface temperature, we can mitigate the impact of variability and gain a more comprehensive understanding4,5. Global heat accumulation in the climate system, resulting from the current positive Earth's Energy Imbalance (EEI) at the top of the atmosphere, is primarily dominated by changes in Global Ocean Heat Content (GOHC)4. GOHC changes account for approximately 90% of the total heat increase in the past fifty years, while land heating, ice melting, and atmospheric warming contribute around 5%, 3%, and 1% respectively6,7,8. Several studies have indicated an increase in the global heat accumulation rate in recent decades, with values rising from 0.50 [0.32 to 0.69] W/m2 during the period 1971-2006 to 0.79 [0.52 to 1.06] W/m2 (90% confidence interval) for the period 2006-2018 (ref.4,6,7,8,9,10,11,12,13,14,15,16,17,18,19,20,21,22 and Fig. 1). Some studies have even suggested a potential doubling of EEI in the last decade compared to the previous one6,17. Figure 1 |
|
|
|
|
| Description | |
| Contents | 6. I go through the lessons offered by paleoclimate in my new book Our Fragile Moment: How Lessons from Earth's Past Can Help Us Survive the Climate Crisis, and I come away with very different conclusions about what we collectively learn from the Cenozoic cooling, the Pliocene, and the Holocene. The collective evidence from the paleoclimate record tells us that climate models have the climate sensitivity (how much warming we can expect for a specified increase in carbon dioxide concentrations) about right, at least for the range of warming we are likely facing (less than 3C/5F given policies already in place). Of course, if we keep greenhouse gas concentrations elevated for centuries, there is the potential for greater amounts of warming as longer-term climate responses kick in. So it is important to think about strategies for carbon drawdown down the road. Here I agree with Hansen and co-authors. But in terms of what we can expect in the decades ahead, there is no reason, based on the collective evidence from the paleoclimate record, to expect a climate trajectory substantially different from what current generation (i.e. IPCC) models predict. And there is no reason that we can't prevent dangerous levels of warming through concerted efforts to decarbonize the global economy. The obstacles, at least at present, are political, not physical or even technological. |
|
|
|
|
| Description | James E. Hansen ABSTRACT
Improved knowledge of glacial-to-interglacial global temperature change implies that fastfeedback equilibrium climate sensitivity (ECS) is 1.2 ± 0.3°C (2s) per W/m2
. Consistent analysis
of temperature over the full Cenozoic era – including “slow” feedbacks by ice sheets and trace
gases – supports this ECS and implies that CO2 was about 300 ppm in the Pliocene and 400 ppm
at transition to a nearly ice-free planet, thus exposing unrealistic lethargy of ice sheet models.
Equilibrium global warming including slow feedbacks for today’s human-made greenhouse gas
(GHG) climate forcing (4.1 W/m2
) is 10°C, reduced to 8°C by today’s aerosols. Decline of
aerosol emissions since 2010 should increase the 1970-2010 global warming rate of 0.18°C per
decade to a post-2010 rate of at least 0.27°C per decade. Under the current geopolitical approach
to GHG emissions, global warming will likely pierce the 1.5°C ceiling in the 2020s and 2°C
before 2050. Impacts on people and nature will accelerate as global warming pumps up
hydrologic extremes. The enormity of consequences demands a return to Holocene-level global
temperature. Required actions include: 1) a global increasing price on GHG emissions, 2) EastWest cooperation in a way that accommodates developing world needs, and 3) intervention with
Earth’s radiation imbalance to phase down today’s massive human-made “geo-transformation”
of Earth’s climate. These changes will not happen with the current geopolitical approach, but
current political crises present an opportunity for reset, especially if young people can grasp their
situation. |
|
|
|
|
Agenda
Doug "“ Does climate sensitivity matter in the short term?
Sev "“ Greenknights "“ putting Buoyant flakes before US Congress
Rebecca "“ MCB Great barrier reef "“ status Stephen?
Doug "“ Beneath the Polar Sun
Doug "“ Kamchatka volcano "“ effects measurement?
Clive "“ Greenland "“ grayland BBC video.
Peter - pictures of Greenland
Mannajo "“ comment on Greenland
Sev "“ Cartoon
Chat
21:01:22 From Sev Clarke : https://www.theguardian.com/commentisfree/2023/apr/17/hey-dont-krill-yourself-humanity?utm_term=643d18a6ed47af625f01d0443e5baa44\u0026utm_campaign=BestOfGuardianOpinionAUS\u0026utm_source=esp\u0026utm_medium=Email\u0026CMP=opinionau_email
21:01:27 From Sev Clarke : https://docs.google.com/document/d/1dpytym6Pq3g1UkrnSLE7zHRxYyimlxfkj7jYGzaX95I/edit
21:15:01 From Doug Grandt (Vermont) : PBS's movie |  (Click HERE to view the transcrript) (Click HERE to view the transcrript) |
|
|
|
|
|
| | # |
Name
(N/A = Not Available) |
Title |
Ref Date |
Date Added |
|---|
|
|
|
| Author | Metta Spencer |
| Description | Project Save the World provides this meeting place for activists and researchers working to save our world from these global problems: war and weapons, global warming, famine, pandemics, radioactive risks, cyber risks, economic, governmental and human rights risks, and civil society risks. We offer you a choice of forums that we’ve produced (watch them as videos, listen as audio podcasts, or read transcripts and summaries), columns to discuss aspects of these nine global dangers, and an events listing for you to publicize upcoming events, and learn about other public activities. |
| Contents | Project Save the World began in 2018 when Professor Metta Spencer, then the outgoing president of Science for Peace and the long-time founder and editor of Peace Magazine, organized a two-day conference, “How to Save the World in a Hurry,” at the University of Toronto. The initial purpose was for progressive NGO leaders to define 25 “planks” in a single “platform” that, if implement together, would greatly reduce the risk of the most serious threats to humankind: war and weapons; global warming; famine; pandemics; radioactive contamination; cyber risks; and the negative effects of economics, governance; and civil society. About 100 persons attended.
On the first day, experts spoke and answered questions about the particular risks in which they specialized. The second day was for breakout groups to discuss these threats. These groups generated the overall Platform for Survival, which was adopted in a plenary session.
As a follow-up, we created a new website, tosavetheworld.ca, and gradually adopted an organizational title: Project Save the World. We acquired a YouTube channel and began holding forums by Zoom — hour-long discussions by experts about these global threats, live on YouTube, and which we edited and posted permanently both on YouTube and our website. We advertise these conversations on social media and by mailed posters to a growing list of frequent viewers.
Although initially Project Save the World was produced and managed as the project of Peace Magazine,
a small publication for peace activists, over time the strength of the two organizations reversed. Peace Magazine’s circulation was declining markedly, whereas the work and expense involved in producing the website and forum series mushroomed. Also, the magazine’s 40-year archive, which is available as text-only on the Internet, attracts a large audience of people searching for particular topics with browsers.
During Covid, the magazine’s printing house went out of business so we began to publish the magazine exclusively in a digital form, available through a magazine aggregating company, PressReader, although the paid subscriptions remain insufficient to predict a bright future as a profit-seeking publication.
However, the digital format made it possible to distribute the magazine free-of-charge to thousands of like-minded organizations worldwide. Just as the prospects of the print magazine vanished, the actual distribution of the digital publication multiplied by the thousands. We were mainly producing articles based on the discussions of researchers in the forum series. In effect, the magazine had morphed into an exceptionally fine house organ, the newsletter of Project Save the World.
Now in 2023, that de facto reversal will be recognized with legal and official changes. CANDIS, the not-for-profit corporation that had owned and controlled Peace Magazine, will turn over the magazine to a newly incorporated not-for-profit, Project Save the World, and then dissolve, having no further purpose. The magazine will fulfill part of Project Save the World’s official purpose, namely “the advancement of education by providing an open forum for qualified researchers to discuss their research with other experts and to make those results available to the public.”
The second half of this purpose — making the results available to the public — is now fulfilled by all three of our platforms; our two websites, the forum of videos and audio podcasts, and our digital newsletter/“Peace Magazine.” These services are all widely available withut charge as our offering toward the survival of humankind. But we thank those subscribers to the magazine who remain with us loyally and still pay, now mainly throughPressReader, CAN $20 per year.
We. the members of Project Save the World, are all people who have supported some of these activities, either by volunteering to work on the publication, a website, or the “broadcasts” or by donating money – usually about CAN $100 — at least once within the past three years. If newcomers wish to participate in these projects, please contact us, for we have a list of chores that sometimes requires volunteer assistance. Email us, stating your interests and aptitudes, at project@tosavetheworld.ca. |
|
|
|
|
| Description | Earth is a cool place
Earth is home to nearly 9 million species of plants and animals, 8 billion humans and surely the most beautiful and diverse nature in the known universe. We can be proud of our incredible planet.
Our planet is in need
Earth is under threat. Global temperatures have risen by around 1.2 degrees since the industrial revolution; the consequences of this warming are already dramatic, but they are minor in comparison to what lies ahead. |
| Contents | The Planet Earth Coolkit
It’s time to act in order to keep the planet cool. Humans can help Earth turn down its thermostat, enhancing its own natural processes to reduce global temperatures. We have grouped these processes together into what we call The Planet Earth Coolkit.
We need you to help us get the message out that global cooling is possible!
Global warming is the biggest threat to mankind. It is time now to Cool Planet Earth. |
|
|
|
|
| Description | Global heating of the Earth system is unequivocal. However, detecting an acceleration of Earth heating has remained elusive to date, despite suggestive evidence of a potential increase in heating rates. In this study, we demonstrate that since 1960, the warming of the world ocean has accelerated at a relatively consistent pace of 0.15 ± 0.05 (W/m2)/decade, while the land, cryosphere, and atmosphere have exhibited an accelerated pace of 0.013 ± 0.003 (W/m2)/decade. This has led to a substantial increase in ocean warming, with a magnitude of 0.91 ± 0.80 W/m2 between the decades 1960-1970 and 2010-2020, which overlies substantial decadal-scale variability in ocean warming of up to 0.6 W/m2. Our findings withstand a wide range of sensitivity analyses and are consistent across different observation-based datasets. The long-term acceleration of Earth warming aligns qualitatively with the rise in CO2 concentrations and the decline in aerosol concentration during the same period, but further investigations are necessary to properly attribute these changes. |
| Contents | In the past 150 years, Earth's climate has been warming at a rate that is unprecedented in at least the last 2000 years1. This human-caused warming has caused widespread adverse impacts and related losses and damages to nature and people, which will continue in the future as global climate continues to warm2. Detecting changes in the rate of warming is crucial for informed decision-making in international climate negotiations, with the aim of limiting global warming to specific levels. However, it remains a significant challenge to detect such changes due to the substantial internal variability of the climate system on a decadal scale (e.g., ref. 3). In this paper, we address this challenge by examining the global heat accumulation rate across the entire climate system, including the ocean, atmosphere, cryosphere, and land. By focusing on this integrated view, rather than solely relying on changes in global mean surface temperature, we can mitigate the impact of variability and gain a more comprehensive understanding4,5. Global heat accumulation in the climate system, resulting from the current positive Earth's Energy Imbalance (EEI) at the top of the atmosphere, is primarily dominated by changes in Global Ocean Heat Content (GOHC)4. GOHC changes account for approximately 90% of the total heat increase in the past fifty years, while land heating, ice melting, and atmospheric warming contribute around 5%, 3%, and 1% respectively6,7,8. Several studies have indicated an increase in the global heat accumulation rate in recent decades, with values rising from 0.50 [0.32 to 0.69] W/m2 during the period 1971-2006 to 0.79 [0.52 to 1.06] W/m2 (90% confidence interval) for the period 2006-2018 (ref.4,6,7,8,9,10,11,12,13,14,15,16,17,18,19,20,21,22 and Fig. 1). Some studies have even suggested a potential doubling of EEI in the last decade compared to the previous one6,17. Figure 1 |
|
|
|
|
| Author | Audrey Minière 1,2*, Karina von Schuckmann 2 , Jean-Baptiste Sallée 3 & LinusVogt 3 |
| Description | Global heating of the Earth system is unequivocal. However, detecting an acceleration of Earth
heating has remained elusive to date, despite suggestive evidence of a potential increase in
heating rates. In this study, we demonstrate that since 1960, the warming of the world ocean has
accelerated at a relatively consistent pace of 0.15± 0.05 (W/m2
)/decade, while the land, cryosphere,
and atmosphere have exhibited an accelerated pace of 0.013± 0.003 (W/m2
)/decade. This has led to
a substantial increase in ocean warming, with a magnitude of 0.91± 0.80 W/m2
between the decades
1960–1970 and 2010–2020, which overlies substantial decadal-scale variability in ocean warming
of up to 0.6 W/m2
. Our fndings withstand a wide range of sensitivity analyses and are consistent
across diferent observation-based datasets. The long-term acceleration of Earth warming aligns
qualitatively with the rise in CO2 concentrations and the decline in aerosol concentration during the
same period, but further investigations are necessary to properly attribute these changes. |
| Contents | In the past 150 years, Earth’s climate has been warming at a rate that is unprecedented in at least the last 2000
years1
. Tis human-caused warming has caused widespread adverse impacts and related losses and damages to
nature and people, which will continue in the future as global climate continues to warm2
. Detecting changes in
the rate of warming is crucial for informed decision-making in international climate negotiations, with the aim
of limiting global warming to specifc levels. However, it remains a signifcant challenge to detect such changes
due to the substantial internal variability of the climate system on a decadal scale (e.g., ref. 3
). In this paper, we
address this challenge by examining the global heat accumulation rate across the entire climate system, including
the ocean, atmosphere, cryosphere, and land. By focusing on this integrated view, rather than solely relying on
changes in global mean surface temperature, we can mitigate the impact of variability and gain a more comprehensive understanding4,5
.
Global heat accumulation in the climate system, resulting from the current positive Earth’s Energy Imbalance
(EEI) at the top of the atmosphere, is primarily dominated by changes in Global Ocean Heat Content (GOHC)4
.
GOHC changes account for approximately 90% of the total heat increase in the past ffy years, while land heating, ice melting, and atmospheric warming contribute around 5%, 3%, and 1% respectively6–8
. Several studies
have indicated an increase in the global heat accumulation rate in recent decades, with values rising from 0.50
[0.32 to 0.69] W/m2
during the period 1971–2006 to 0.79 [0.52 to 1.06] W/m2
(90% confdence interval) for the
period 2006–2018 (ref.4,6–22 and Fig. 1). Some studies have even suggested a potential doubling of EEI in the last
decade compared to the previous one6,17.
Despite this suggestive body of evidence, no study has conducted an analysis of heat accumulation acceleration since 1960 to date. While the results presented in Fig. 1 provide insights, they represent accumulation rates
computed over varying time spans, with higher rates calculated over decadal periods and lower rates calculated
over multi-decadal periods. Tis variation in time spans makes it challenging to make defnitive and quantifed
statements about acceleration. Additionally, the use of diverse datasets and methodologies can signifcantly
impact the calculated accumulation rates. Te only notable climate variable where acceleration has previously
been detected in past decades is Global Mean Sea Level (GMSL)23–31. Tis GMSL acceleration has been attributed
to factors such as increasing GOHC leading to thermal expansion of seawater, declining land water storage, or
increasing land ice melt25–27,31,32.
In this paper, we present the frst observation-based quantifcation of the acceleration of Earth’s system heating. Our study adopts a systematic approach, incorporating multiple datasets and employing various methods.
We estimate the rate of change and acceleration of Earth’s heat content using a collection of GOHC time series |
|
|
|
|
| Author | Michael MacCracken, Suzanne Reed |
| Description | Official reports on the state of the climate and progress toward meeting the 2015 Paris Agreement 1.5°C and 2.0°C targets for limiting global warming set the stage for COP28 opening on November 30. The findings were alarming to many, but apparently, not alarming enough for negotiators gathered in Dubai to accept reality. Bottom line, the temperature is rising at an accelerating pace and progress toward meeting the Paris targets is sorely lagging. It may not be as bad as it could have been if we did nothing, but that is a poor excuse for COP28 refusing to admit that the current global climate strategy is an epic fail. |
| Contents | Central to the continuing failure of the COP process is the sole reliance on reducing greenhouse gas (GHG) emissions and removing accumulated emissions from the atmosphere to limit temperature rise. A much broader approach is urgently needed to restore and stabilize climate conditions that can sustain all life forms. It is time to integrate climate cooling into a credible global strategy.
Climate change and the severity of its impacts are increasing much faster than calculated by various models and analyses projecting future conditions. What’s more, the impacts are interacting to push critical ecological, hydrologic, and glacial systems over thresholds, known as tipping points, thus amplifying climate disasters. This truth is being told by current events, not speculative assumptions. The reports released to inform COP28 deliberations confirm 2023 as the worst year yet of climate change with the Global South and vulnerable populations around the world bearing the brunt. |
|
|
|
|
|
| | # |
Name
(N/A = Not Available) |
Title |
Ref Date |
Date Added |
|---|
|
|
|
| Description | |
| Contents | 6. I go through the lessons offered by paleoclimate in my new book Our Fragile Moment: How Lessons from Earth's Past Can Help Us Survive the Climate Crisis, and I come away with very different conclusions about what we collectively learn from the Cenozoic cooling, the Pliocene, and the Holocene. The collective evidence from the paleoclimate record tells us that climate models have the climate sensitivity (how much warming we can expect for a specified increase in carbon dioxide concentrations) about right, at least for the range of warming we are likely facing (less than 3C/5F given policies already in place). Of course, if we keep greenhouse gas concentrations elevated for centuries, there is the potential for greater amounts of warming as longer-term climate responses kick in. So it is important to think about strategies for carbon drawdown down the road. Here I agree with Hansen and co-authors. But in terms of what we can expect in the decades ahead, there is no reason, based on the collective evidence from the paleoclimate record, to expect a climate trajectory substantially different from what current generation (i.e. IPCC) models predict. And there is no reason that we can't prevent dangerous levels of warming through concerted efforts to decarbonize the global economy. The obstacles, at least at present, are political, not physical or even technological. |
|
|
|
| 2 |
acp.copernicus.org/articl... |
Ice melt, sea level rise and superstorms: evidence from paleoclimate data, climate modeling, and modern observations that 2 ?C global warming could be dangerous |
3/22/26 |
1/6/24 |
|
|
|
| 2 |
acp.copernicus.org/articl... |
Ice melt, sea level rise and superstorms: evidence from paleoclimate data, climate modeling, and modern observations that 2 ?C global warming could be dangerous |
3/22/26 |
1/6/24 |
|
| Author | James Hansen1 , Makiko Sato1 , Paul Hearty2 , Reto Ruedy3,4 , Maxwell Kelley3,4 , Valerie Masson-Delmotte5 , Gary Russell4 , George Tselioudis4 , Junji Cao6 , Eric Rignot7,8 , Isabella Velicogna7,8 , Blair Tormey9 , Bailey Donovan10 , Evgeniya Kandiano11, Karina von Schuckmann12, Pushker Kharecha1,4 , Allegra N. Legrande4 , Michael Bauer4,13 , and Kwok-Wai Lo3,4 |
| Description | Abstract. We use numerical climate simulations, paleoclimate data, and modern observations to study the effect of
growing ice melt from Antarctica and Greenland. Meltwater tends to stabilize the ocean column, inducing amplifying
feedbacks that increase subsurface ocean warming and ice
shelf melting. Cold meltwater and induced dynamical effects
cause ocean surface cooling in the Southern Ocean and North
Atlantic, thus increasing Earth’s energy imbalance and heat
flux into most of the global ocean’s surface. Southern Ocean
surface cooling, while lower latitudes are warming, increases
precipitation on the Southern Ocean, increasing ocean stratification, slowing deepwater formation, and increasing ice
sheet mass loss. These feedbacks make ice sheets in contact
with the ocean vulnerable to accelerating disintegration. We
hypothesize that ice mass loss from the most vulnerable ice,
sufficient to raise sea level several meters, is better approximated as exponential than by a more linear response. Doubling times of 10, 20 or 40 years yield multi-meter sea level
rise in about 50, 100 or 200 years. Recent ice melt doubling
times are near the lower end of the 10–40-year range, but
the record is too short to confirm the nature of the response.
The feedbacks, including subsurface ocean warming, help
explain paleoclimate data and point to a dominant Southern
Ocean role in controlling atmospheric CO2, which in turn exercised tight control on global temperature and sea level. The
millennial (500–2000-year) timescale of deep-ocean ventilation affects the timescale for natural CO2 change and thus
the timescale for paleo-global climate, ice sheet, and sea |
|
|
|
|
|
| | # |
Name
(N/A = Not Available) |
Title |
Ref Date |
Date Added |
|---|
|
|
| 1 |
essopenarchive.org/doi/fu... |
Exploring potential atmospheric methane removal approaches: an example research roadmap for chlorine radical enhancement |
11/8/23 |
11/8/23 |
|
|
|
| 1 |
essopenarchive.org/doi/fu... |
Exploring potential atmospheric methane removal approaches: an example research roadmap for chlorine radical enhancement |
11/8/23 |
11/8/23 |
|
| Description | The escalating climate crisis requires rapid action to reduce the concentrations of atmospheric greenhouse gases and lower global surface temperatures. Methane will play a critical role in near-term warming due to its high radiative forcing and short |
| Contents | The escalating climate crisis requires rapid action to reduce the concentrations of atmospheric greenhouse gases and lower global surface temperatures. Methane will play a critical role in near-term warming due to its high radiative forcing and short atmospheric lifetime. Methane emissions have accelerated in recent years and there is significant risk and uncertainty associated with the future growth in natural emissions. The largest natural sink of methane occurs through oxidation reactions with atmospheric hydroxyl and chlorine radicals. Enhanced atmospheric oxidation could be a potential approach to remove atmospheric methane. One method proposes the addition of iron salt aerosols (ISA) to the atmosphere, mimicking a natural process that is proposed to occur when mineral dust mixes with chloride from sea spray to form iron chlorides, which are photolyzed by sunlight to produce chlorine radicals. Under the right conditions, lofting ISA into the atmosphere could potentially reduce atmospheric methane concentrations and lower global surface temperatures. Recognizing that potential atmospheric methane removal must only be considered as an additive measure-in addition to, not replacing, crucial anthropogenic greenhouse gas emission reductions and carbon dioxide removal-roadmaps can be a valuable tool to organize and streamline interdisciplinary and multifaceted research to efficiently move towards an understanding of whether an approach may be viable and socially acceptable, or if it is nonviable and further research should be deprioritized. Here we present an example of a five-year research roadmap to explore whether ISA enhancement of the chlorine radical sink could be a viable and socially acceptable atmospheric methane removal approach. |
|
|
|
|
| Description |
Marine Cloud Brightening
Marine cloud brightening refers to an albedo modification technique that aims to increase the reflectivity, and possibly even the lifetimes, of certain clouds in order to reflect more sunlight back into space and partially offset some of the impacts of climate change. The most common proposal for achieving such a goal is to inject naturally occurring sea salt into cloud updrafts. But a variety of methods are being researched. |
| Contents | For example, even if marine cloud brightening could work, it could affect large scale climate and weather patterns if it were used on a large enough scale, say to achieve a level of “radiative forcing” that would be big enough to offset some of the greatest impacts of climate change. (This is because marine cloud brightening could only be implemented in limited areas, where the right kinds of clouds exist, which is perhaps only 10 percent of the planet’s surface.) There are several key risks that need to be better understood.
Still, the fact that more research is needed is true for all geoengineering techniques. So even though the scientists within our group focus most closely on stratospheric aerosol injection, we believe that MCB research is valuable. |
|
|
|
|
Agenda
John N – BBC News says 1.5C will arrive twice as soon as previously thought.
- Letter to Guardian re Hansen Global warming in pipeline paper
Chris V – Report from Chatham House – focusing on 1.5 is flawed, focus on tipping points
Hugh H – MCB and controlling hurricanes
Chris V – Compendium of interventions – in the chat
Sev – NOAC website progress – integration of Blue Cooling Initiative, HPAC ,PRAG
Ron – Comments on bunker fuel letter?
Clive – Just Have A Think – recent video very good.
– Radiative forcing to -0.26 W/m2 waterfall chart demo.
Chat
20:06:23 From Chris Vivian - GESAMP WG 41 : Carbon emissions threaten 1.5C climate threshold sooner than thought – report - https://www.bbc.co.uk/news/science-environment-67242386
20:07:16 From Chris Vivian - GESAMP WG 41 : Global warming: Why focusing on 1.5C is flawed - https://www.chathamhouse.org/publications/the-world-today/2023-10/global-warming-why-focusing-15c-flawed?utm_source=Chatham%20House&utm_medium=email&utm_campaign=14169358_CH%20-%20Content%20Newsletter%20-%2017.10.23&utm_content=Global-Title&dm_i=1S3M,8FP5A,NODY6,YTVON,1
20:10:08 From Chris Vivian - GESAMP WG 41 : FROZEN ARCTIC: Compendium of interventions to slow down, halt, and reverse the effects of climate change in the Arctic and northern regions - https://new.uarctic.org/media/to0bjpal/frozen-arctic-rra-compendium.pdf
20:10:48 From Ron Baiman : First part of Sharpe's book is very good on risk analysis and climate.
20:12:43 From Ron Baiman : Bunker fuel letter link: https://docs.google.com/document/d/1WNsRI8GbyZgdso39ptKuKFI2HA6ZuBni/edit?usp=sharing&ouid=116465941111195452408&rtpof=true'sd=true
20:13:23 From Herb Simmens : The Nov 16th HPAC meeting will feature Mike McCracken discussing the differing approaches to addressing risk that various professions and institutions take
20:15:28 From Herb Simmens : This Thursday Nov 2 HPAC meeting at 3:30 PM EDT 19:30 PM GMT will feature Doug MacMartin of Cornell on SRM/SAI..
20:17:00 From Robert Chris : https://www.nature.com/articles/s41558-023-01848-5
20:19:42 From Herb Simmens : This blog post has a link to the paper in the first sentence https://scienceisshiny.wordpress.com/2023/10/30/carbon-budgets-how-hard-is-the-paris-agreement-now/
20:21:37 From Bill Chapman, Brooklyn : I was horrified when Trump and some Republicans started talking about |  (Click HERE to view the transcrript) (Click HERE to view the transcrript) |
|
|
|
| 4 |
essd.copernicus.org/artic... |
ESSD - Indicators of Global Climate Change 2022: annual update of large-scale indicators of the state of the climate system and human influence |
6/8/23 |
11/5/23 |
|
|
|
| 4 |
essd.copernicus.org/artic... |
ESSD - Indicators of Global Climate Change 2022: annual update of large-scale indicators of the state of the climate system and human influence |
6/8/23 |
11/5/23 |
|
| Description | Abstract. Intergovernmental Panel on Climate Change (IPCC) assessments are the trusted source of scientific evidence for climate negotiations taking place under the United Nations Framework Convention on Climate Change (UNFCCC), including the first global stocktake under the Paris Agreement that will conclude at COP28 in December 2023. Evidence-based decision-making needs to be informed by up-to-date and timely information on key indicators of the state of the climate system and of the human influence on the global climate system. However, successive IPCC reports are published at intervals of 5-10 years, creating potential for an information gap between report cycles. We follow methods as close as possible to those used in the IPCC Sixth Assessment Report (AR6) Working Group One (WGI) report. We compile monitoring datasets to produce estimates for key climate indicators related to forcing of the climate system: emissions of greenhouse gases and short-lived climate forcers, greenhouse gas concentrations, radiative forcing, surface temperature changes, the Earth's energy imbalance, warming attributed to human activities, the remaining carbon budget, and estimates of global temperature extremes. The purpose of this effort, grounded in an open data, open science approach, is to make annually updated reliable global climate indicators available in the public domain (https://doi.org/10.5281/zenodo.8000192, Smith et al., 2023a). As they are traceable to IPCC report methods, they can be trusted by all parties involved in UNFCCC negotiations and help convey wider understanding of the latest knowledge of the climate system and its direction of travel. The indicators show that human-induced warming reached 1.14 [0.9 to 1.4]?°C averaged over the 2013-2022 decade and 1.26 [1.0 to 1.6]?°C in 2022. Over the 2013-2022 period, human-induced warming has been increasing at an unprecedented rate of over 0.2?°C per decade. This high rate of warming is caused by a combination of greenhouse gas emissions being at an all-time high of 54?±?5.3?GtCO2e over the last decade, as well as reductions in the strength of aerosol cooling. Despite this, there is evidence that increases in greenhouse gas emissions have slowed, and depending on societal choices, a continued series of these annual updates over the critical 2020s decade could track a change of direction for human influence on climate. |
| Contents | Increased greenhouse gas concentrations combined with reductions in aerosol pollution have led to rapid increases in human-induced effective radiative forcing, which has in turn led to atmosphere, land, cryosphere and ocean warming (Gulev et al., 2021). This in turn has led to an intensification of many weather and climate extremes, particularly more frequent and more intense hot extremes, and heavy precipitation across most regions of the world (Seneviratne et al., 2021). Given the speed of recent change, and the need for evidence-based decision-making, this Indicators of Global Climate Change (IGCC) update assembles the latest scientific understanding on the current state and evolution of the climate system and of human influence to support policymakers whilst the next Intergovernmental Panel on Climate Change (IPCC) assessment is under preparation. This first annual update is focused on indicators related to heating of the climate system, building from greenhouse gas emissions towards estimates of human-induced warming and the remaining carbon budget. In future years, this effort could be expanded to encompass other indicators, including global precipitation changes and related extremes. We adopt the Global Carbon Budget ethos of a community-wide inclusive effort that synthesises work from across a large and diverse global scientific community in a timely fashion (Friedlingstein et al., 2022a). Like the Global Carbon Budget, this initiative arises from the international science community to establish a knowledge base to support policy debate and action to meet the Paris Agreement temperature goal. This update complements other international efforts under the auspices of the Global Climate Observing System (GCOS) and the World Meteorological Organization (WMO). Annual state-of-the-climate reports are released by the WMO which use much of the same data analysed here for surface temperature and energy budget trends. The Bulletin of American Meteorological Society (BAMS) releases annual state-of-the-climate reports covering many essential variables including temperature and greenhouse gas concentrations. However, these reports focus on statistics from the previous year and make slightly different choices over datasets and analysis compared to the IPCC (see Sect. 5). The Global Carbon Project publishes updated carbon dioxide datasets which are used directly in this report. There is no similarly structured activity that provides all the necessary datasets to update the assessment of human influence on global surface temperature annually. The update is based on methodologies for key climate indicators assessed by the IPCC Sixth Assessment Report (AR6) of the physical science basis of climate change (Working Group One (WGI) report; IPCC, 2021a) as well as Chap. 2 of the WGIII report (Dhakal et al., 2022) and is aligned with the efforts initiated in AR6 to implement FAIR (Findable, Accessible, Interoperable, Reusable) principles for reproducibility and reusability (Pirani et al., 2022; Iturbide et al., 2022). IPCC reports make a much wider assessment of the science and methodologies - we do not attempt to reproduce the comprehensive nature of these IPCC assessments here. The IPCC Special Report on Global Warming of 1.5?°C (SR1.5), published in 2018, provided an assessment of the level of human-induced warming and cumulative emissions to date (Allen et al., 2018) and the remaining carbon budget (Rogelj et al., 2018) to support the evidence base on how the world is progressing in terms of meeting aspects of the Paris Agreement. The AR6 WGI Report, published in 2021, assessed past, current and future changes of these and other key global climate indicators, as well as undertaking an assessment of the Earth's energy budget. It also updated its approach for estimating human-induced warming and global warming level. In AR6 WGI and here, reaching a level of global warming is defined as the global surface temperature change, averaged over a 20-year period, exceeding a particular level of global warming, for example, 1.5?°C global warming. Given the current rates of change and the likelihood of reaching 1.5?°C of global warming in the first half of the 2030s (Lee et al., 2021, 2023; Riahi et al., 2022), it is important to have robust, trusted and also timely climate indicators in the public domain to form an evidence base for effective science-based decision-making. When making their assessments, authors of IPCC reports assess published literature but also apply established published analysis methods to assessed datasets, such as the dataset produced by the latest climate model intercomparison projects (Lee et al., 2021). The authors combine and analyse both model and observational data as part of their expert assessment, making assessments of the trustworthiness and error characteristics of different datasets. It is this synthetic analysis by IPCC authors that derives the estimates of key climate indicators. Wherever possible, these same assessed methodological approaches are implemented here to provide the updates with variations clearly flagged and documented. The same approach, using the same datasets (updated by 2 years) and methods as employed in WGI, was used in the AR6 Synthesis Report (2023) (AR6 SYR; Lee et al., 2023) to provide an updated assessment of the latest atmospheric well-mixed greenhouse gas concentrations (up to 2021) and decadal average change in global surface temperature (+1.15?°C [1.00-1.25?°C] in 2013-2022 for global surface temperature). However, the assessment of human-induced warming was not updated (and therefore only covers warming up to the decade 2010-2019), nor was the remaining carbon budget updated, so the related information in the AR6 SYR report remained based on data up to the end of 2019. The indicators in this first annual update give important insights into the magnitude and the pace of global warming. This paper provides the basis for a dashboard of climate indicators grounded in IPCC methodologies and directly traceable to reports published as part of the AR6 cycle. We employ datasets that can be updated on a regular basis between the publication of IPCC reports. Note that there are other similar initiatives underway to update other AR6 cycle products; for example, the evolution of the WGI Interactive Atlas (Gutiérrez et al., 2021) is being developed under the Copernicus Climate Change Service (C3S) and has potential connections and synergies with this initiative that will be explored in the future. Our longer-term ambition is to rigorously track both climate system change and methodological improvements between IPCC report cycles, thereby building consistency and awareness. An example of why tracking methodological change is important was the updated estimate for historic warming (the increase in global surface temperature from 1850-1900 to 1986-2005). This was 0.08 [-0.01 to 0.12]?°C higher in the AR6 than in the fifth assessment report (AR5) and SR1.5. Datasets and methods of evaluating global temperature changes altered between the AR5 and AR6, leading to a small shift in the historical temperature. This was reflected in changes between AR5 and AR6, whereas SR1.5 mostly relied on methodologies from AR5 (see AR6 WGI Cross Chap. Box 2.3, Gulev et al., 2021). Annual updates provide indications of possible future methodological shifts that subsequent IPCC reports may make as science advances and can detail their impact on perceived trends. The update is organised as follows: emissions (Sect. 2) and greenhouse gas (GHG) concentrations (Sect. 3) are used to develop updated estimates of effective radiative forcing (Sect. 4). Observations of global surface temperature change (Sect. 5) and Earth's energy imbalance (Sect. 6) are key global indicators of a warming world. The global surface temperature change is formally attributed to human activity in Sect. 7, which tracks human-induced warming. Section 8 updates the remaining carbon budget to policy-relevant temperature thresholds. Section 9 gives an example of global-scale indicators associated with climate extremes of maximum land surface temperatures. An important purpose of the exercise is to make these indicators widely available and understood. Plans for a web dashboard are discussed in Sect. 10 and code and data availability in Sect. 11, and conclusions are presented in Sect. 12. Data are available at https://doi.org/10.5281/zenodo.8000192 (Smith et al., 2023a). |
|
|
|
| 5 |
essd.copernicus.org/artic... |
ESSD - Indicators of Global Climate Change 2022: annual update of large-scale indicators of the state of the climate system and human influence |
6/8/23 |
11/8/23 |
|
|
|
| 5 |
essd.copernicus.org/artic... |
ESSD - Indicators of Global Climate Change 2022: annual update of large-scale indicators of the state of the climate system and human influence |
6/8/23 |
11/8/23 |
|
| Description | Abstract. Intergovernmental Panel on Climate Change (IPCC) assessments are the trusted source of scientific evidence for climate negotiations taking place under the United Nations Framework Convention on Climate Change (UNFCCC), including the first global stocktake under the Paris Agreement that will conclude at COP28 in December 2023. Evidence-based decision-making needs to be informed by up-to-date and timely information on key indicators of the state of the climate system and of the human influence on the global climate system. However, successive IPCC reports are published at intervals of 5-10 years, creating potential for an information gap between report cycles. We follow methods as close as possible to those used in the IPCC Sixth Assessment Report (AR6) Working Group One (WGI) report. We compile monitoring datasets to produce estimates for key climate indicators related to forcing of the climate system: emissions of greenhouse gases and short-lived climate forcers, greenhouse gas concentrations, radiative forcing, surface temperature changes, the Earth's energy imbalance, warming attributed to human activities, the remaining carbon budget, and estimates of global temperature extremes. The purpose of this effort, grounded in an open data, open science approach, is to make annually updated reliable global climate indicators available in the public domain (https://doi.org/10.5281/zenodo.8000192, Smith et al., 2023a). As they are traceable to IPCC report methods, they can be trusted by all parties involved in UNFCCC negotiations and help convey wider understanding of the latest knowledge of the climate system and its direction of travel. The indicators show that human-induced warming reached 1.14 [0.9 to 1.4]?°C averaged over the 2013-2022 decade and 1.26 [1.0 to 1.6]?°C in 2022. Over the 2013-2022 period, human-induced warming has been increasing at an unprecedented rate of over 0.2?°C per decade. This high rate of warming is caused by a combination of greenhouse gas emissions being at an all-time high of 54?±?5.3?GtCO2e over the last decade, as well as reductions in the strength of aerosol cooling. Despite this, there is evidence that increases in greenhouse gas emissions have slowed, and depending on societal choices, a continued series of these annual updates over the critical 2020s decade could track a change of direction for human influence on climate. |
| Contents | Increased greenhouse gas concentrations combined with reductions in aerosol pollution have led to rapid increases in human-induced effective radiative forcing, which has in turn led to atmosphere, land, cryosphere and ocean warming (Gulev et al., 2021). This in turn has led to an intensification of many weather and climate extremes, particularly more frequent and more intense hot extremes, and heavy precipitation across most regions of the world (Seneviratne et al., 2021). Given the speed of recent change, and the need for evidence-based decision-making, this Indicators of Global Climate Change (IGCC) update assembles the latest scientific understanding on the current state and evolution of the climate system and of human influence to support policymakers whilst the next Intergovernmental Panel on Climate Change (IPCC) assessment is under preparation. This first annual update is focused on indicators related to heating of the climate system, building from greenhouse gas emissions towards estimates of human-induced warming and the remaining carbon budget. In future years, this effort could be expanded to encompass other indicators, including global precipitation changes and related extremes. We adopt the Global Carbon Budget ethos of a community-wide inclusive effort that synthesises work from across a large and diverse global scientific community in a timely fashion (Friedlingstein et al., 2022a). Like the Global Carbon Budget, this initiative arises from the international science community to establish a knowledge base to support policy debate and action to meet the Paris Agreement temperature goal. This update complements other international efforts under the auspices of the Global Climate Observing System (GCOS) and the World Meteorological Organization (WMO). Annual state-of-the-climate reports are released by the WMO which use much of the same data analysed here for surface temperature and energy budget trends. The Bulletin of American Meteorological Society (BAMS) releases annual state-of-the-climate reports covering many essential variables including temperature and greenhouse gas concentrations. However, these reports focus on statistics from the previous year and make slightly different choices over datasets and analysis compared to the IPCC (see Sect. 5). The Global Carbon Project publishes updated carbon dioxide datasets which are used directly in this report. There is no similarly structured activity that provides all the necessary datasets to update the assessment of human influence on global surface temperature annually. The update is based on methodologies for key climate indicators assessed by the IPCC Sixth Assessment Report (AR6) of the physical science basis of climate change (Working Group One (WGI) report; IPCC, 2021a) as well as Chap. 2 of the WGIII report (Dhakal et al., 2022) and is aligned with the efforts initiated in AR6 to implement FAIR (Findable, Accessible, Interoperable, Reusable) principles for reproducibility and reusability (Pirani et al., 2022; Iturbide et al., 2022). IPCC reports make a much wider assessment of the science and methodologies - we do not attempt to reproduce the comprehensive nature of these IPCC assessments here. The IPCC Special Report on Global Warming of 1.5?°C (SR1.5), published in 2018, provided an assessment of the level of human-induced warming and cumulative emissions to date (Allen et al., 2018) and the remaining carbon budget (Rogelj et al., 2018) to support the evidence base on how the world is progressing in terms of meeting aspects of the Paris Agreement. The AR6 WGI Report, published in 2021, assessed past, current and future changes of these and other key global climate indicators, as well as undertaking an assessment of the Earth's energy budget. It also updated its approach for estimating human-induced warming and global warming level. In AR6 WGI and here, reaching a level of global warming is defined as the global surface temperature change, averaged over a 20-year period, exceeding a particular level of global warming, for example, 1.5?°C global warming. Given the current rates of change and the likelihood of reaching 1.5?°C of global warming in the first half of the 2030s (Lee et al., 2021, 2023; Riahi et al., 2022), it is important to have robust, trusted and also timely climate indicators in the public domain to form an evidence base for effective science-based decision-making. When making their assessments, authors of IPCC reports assess published literature but also apply established published analysis methods to assessed datasets, such as the dataset produced by the latest climate model intercomparison projects (Lee et al., 2021). The authors combine and analyse both model and observational data as part of their expert assessment, making assessments of the trustworthiness and error characteristics of different datasets. It is this synthetic analysis by IPCC authors that derives the estimates of key climate indicators. Wherever possible, these same assessed methodological approaches are implemented here to provide the updates with variations clearly flagged and documented. The same approach, using the same datasets (updated by 2 years) and methods as employed in WGI, was used in the AR6 Synthesis Report (2023) (AR6 SYR; Lee et al., 2023) to provide an updated assessment of the latest atmospheric well-mixed greenhouse gas concentrations (up to 2021) and decadal average change in global surface temperature (+1.15?°C [1.00-1.25?°C] in 2013-2022 for global surface temperature). However, the assessment of human-induced warming was not updated (and therefore only covers warming up to the decade 2010-2019), nor was the remaining carbon budget updated, so the related information in the AR6 SYR report remained based on data up to the end of 2019. The indicators in this first annual update give important insights into the magnitude and the pace of global warming. This paper provides the basis for a dashboard of climate indicators grounded in IPCC methodologies and directly traceable to reports published as part of the AR6 cycle. We employ datasets that can be updated on a regular basis between the publication of IPCC reports. Note that there are other similar initiatives underway to update other AR6 cycle products; for example, the evolution of the WGI Interactive Atlas (Gutiérrez et al., 2021) is being developed under the Copernicus Climate Change Service (C3S) and has potential connections and synergies with this initiative that will be explored in the future. Our longer-term ambition is to rigorously track both climate system change and methodological improvements between IPCC report cycles, thereby building consistency and awareness. An example of why tracking methodological change is important was the updated estimate for historic warming (the increase in global surface temperature from 1850-1900 to 1986-2005). This was 0.08 [-0.01 to 0.12]?°C higher in the AR6 than in the fifth assessment report (AR5) and SR1.5. Datasets and methods of evaluating global temperature changes altered between the AR5 and AR6, leading to a small shift in the historical temperature. This was reflected in changes between AR5 and AR6, whereas SR1.5 mostly relied on methodologies from AR5 (see AR6 WGI Cross Chap. Box 2.3, Gulev et al., 2021). Annual updates provide indications of possible future methodological shifts that subsequent IPCC reports may make as science advances and can detail their impact on perceived trends. The update is organised as follows: emissions (Sect. 2) and greenhouse gas (GHG) concentrations (Sect. 3) are used to develop updated estimates of effective radiative forcing (Sect. 4). Observations of global surface temperature change (Sect. 5) and Earth's energy imbalance (Sect. 6) are key global indicators of a warming world. The global surface temperature change is formally attributed to human activity in Sect. 7, which tracks human-induced warming. Section 8 updates the remaining carbon budget to policy-relevant temperature thresholds. Section 9 gives an example of global-scale indicators associated with climate extremes of maximum land surface temperatures. An important purpose of the exercise is to make these indicators widely available and understood. Plans for a web dashboard are discussed in Sect. 10 and code and data availability in Sect. 11, and conclusions are presented in Sect. 12. Data are available at https://doi.org/10.5281/zenodo.8000192 (Smith et al., 2023a). |
|
|
|
|
|
| | # |
Name
(N/A = Not Available) |
Title |
Ref Date |
Date Added |
|---|
|
|
| 1 |
rebrighten.org/ |
Reflecting Sunlight to Cool the Ocean Surface and Reduce Global Warming |
11/1/23 |
11/14/23 |
|
|
|
| 1 |
rebrighten.org/ |
Reflecting Sunlight to Cool the Ocean Surface and Reduce Global Warming |
11/1/23 |
11/14/23 |
|
| Description | Rebrighten.org is a not for profit organization newly established to study, promote and deploy Marine Cloud Brightening (MCB) as the most effective, safe and rapid available method to reverse global warming. We seek to raise USD $5 million to implement our proposal to prove the feasibility of MCB as a way to cool and re-brighten the planet, alongside broader existing efforts to mitigate warming by cutting and removing greenhouse gases. Help us create a brighter, safer future for the planet & humanity by supporting this crucial project. |
| Contents | What is Marine Cloud Brightening and Why is it necessary?
Marine Cloud Brightening (MCB) is potentially the first feasible way to start cooling the planet by reflecting more sunlight back to space.
Deployment would help slow global temperature rise, refreeze the poles and mitigate extreme weather such as storms, fires, droughts, heatwaves and floods.
Sea salt sprayed into the lower atmosphere in targeted areas is expected to prove a harmless way to reverse warming.
MCB has potential to reverse sea level rise with benefit to cost ratio estimated at 50,000 to 1.
MCB has been recognised for 50 years as a simple way to cool the oceans. It relies on the ‘Twomey Effect’, from its discoverer Dr Sean Twomey, who showed that clouds with smaller drops reflect more sunlight and are brighter than clouds with larger drops. Dr John Latham then showed that the optimal cloud drop size for MCB can be produced with sea salt particles smaller than one micron. |
|
|
|
|
| Description | West Antarctica is headed for decades of rapid melting no matter how quickly humans cut greenhouse gas emissions, and 2023 shattered records for missing sea ice around the continent. |
| Contents | A trio of new scientific analyses about the loss of ice in Antarctica paint a picture of a continent in trouble. Sea ice is disappearing, gigantic portions of the West Antarctic ice sheet are crumbling and even relatively stable East Antarctica is showing worrying changes. |
|
|
|
|
| Description | Carbon dioxide receives a lot of attention because it is warming the climate. However, we think it’s also important to understand the cooling processes that occur naturally in the troposphere. For example:
What is the process that removes powerful greenhouse warming agents such as methane and soot from the air?
What makes bright clouds form in the air?
Moreover, could these processes be safely increased to delay or even reverse today’s melting of Earth’s polar ice sheets? If so, they could prevent the catastrophic effects of carbon dioxide not being drawn down fast enough. Our solutions aim to do that using the same or similar methods that have been operating naturally in the troposphere for millions of years. |
| Contents | Around 90% of the extra heat absorbed by the Earth from climate warming has been taken up by the oceans. Today this is equivalent to 7 Hiroshima bombs exploding in them every second. However, today’s sharp rise in sea surface temperatures is of particular concern to climate scientists and oceanographers. As these warmer seas naturally flow into the polar regions they melt the ice there, sometimes from beneath the ice sheets. In addition, more evaporation puts more water vapour in the air, and water vapour itself acts as a greenhouse gas. Higher humidity in the dark polar winters also produces thick clouds that trap even more heat. This slows the rate of polar ice refreezing, accelerating sea level rise.
The Opportunity
Around 23% of the sun’s energy is reflected away from the Earth by clouds. Typically, only around 40% of the tropical and subtropical oceans are covered by cloud at any time. Increasing cloud cover in these regions by 3-4% could provide sufficient cooling to halt today’s warming trend.
Where do marine clouds come from? Phytoplankton, seaweed, and corals produce the ‘smell of the sea’ (dimethyl sulphide, or DMS). This substance reacts and combines with airborne sea salt and mineral dust to produce microscopic particles known as cloud condensation nuclei (CCN). These CCN particles float naturally in the air, and water vapour naturally condenses onto them up high where the air is cold, forming clouds in the sky. The more DMS there is in the air over the oceans, the brighter the clouds, the longer they last before ‘burning off’ in the sun, and the greater their total cooling effect. |
|
|
|
|
Agenda
Chris – COP27
Brian - Grand XPRIZE for carbon removal
Sev – document
John N – paper
20:07:17 From DV Henkel-Wallace : Alliance of Small Island States www.aosis.org
20:14:42 From Dr Brian von Herzen : Aside from PEM membranes, why go from ch4 to h2?
20:15:26 From Dr Brian von Herzen : Wouldn’t green methane be far more valuable?
20:16:07 From Sev Clarke : because the nanocabon co-product would reduce the h2 cost
20:17:38 From Dr Brian von Herzen : But at energy storage cost
20:17:44 From Dr Brian von Herzen : And at energy loss per m3
20:19:02 From Dr Brian von Herzen : Hawaii has hydrogen injection up to 50% over the past decade
20:19:26 From Sev Clarke : you store the methane at low cost then use renewable energy to generate on site on demand bliue green hydrogen
20:19:50 From Dr Brian von Herzen : Or just use the methane directly?
20:21:01 From Sev Clarke : but not to burn as that releases ghg emissions
20:22:33 From Dr Brian von Herzen : Read “moving to higher ground†for a recent discussion of having to abandon islands-
20:26:30 From Chris Vivian : This Guardian article is interesting as I don’t think
Melting point: could ‘cloud brightening’ slow the thawing of the Arctic?
https://www.theguardian.com/environment/2022/nov/27/melting-point-could-cloud-brightening-slow-the-thawing-of-the-arctic?CMP=Share_iOSApp_Other
20:29:27 From Dr Brian von Herzen : If you go down 1000 meters, the rise in temperature is much smaller, no?
20:32:41 From Robert Tulip : A brighter planet could increase the ice mass of the cryosphere to reverse sea level rise at century time scale.
20:33:06 From Tara Vamos : Tara@ItsAllAboutMovement.com
20:33:44 From Dr Brian von Herzen : 1 @robert
20:43:19 From Dr Brian von Herzen : First problem is buoyancy?
20:46:07 From Dr Brian von Herzen : Cooling the planet- deep cycling results in primary production, resulting in DMS production ,resulting in marine cloud brightening!
20:50:20 From Dr Brian von Herzen : That didn’t stop Greenpeace from suing victor Smetachek in high court in Germany under the London Protocol presumably?
20:51:07 From Dr Brian von Herzen : bicatch
20:53:33 From Dr Brian von Herzen : .06 ignores specular reflection?
21:07:28 From Sev Clarke : no it does not. see how it ws calculated
21:09:53 From Zoom user : Great document Sev. Some of your images with each might help to convey the concepts.
21:11:10 From Chris Vivian : See this paper about MCB: 'Marine cloud brightening; as effective without clouds' - https://acp.copernicus.org/articles/17/13071/2017/acp-17-13071-2017.html
21:13:23 From Zoom user : https://arcticdata.io/catalog/portals/ArcticTides
21:18:04 From Sev Clarke : My Moresolsv4 document provides the graphics
21:18:41 From Chris Vivian : Arctic tidal atlas https://www.nature.com/articles/s41597-020-00578-z |  (Click HERE to view the transcrript) (Click HERE to view the transcrript) |
|
|
|
|
Agenda
Sev – attacks on climate restoration proposers
Chris – Sinking seaweed is beyond the science and ethics
Brian – update on field trials – marine permaculture
Sev – Antarctic ice – worsening – biggest shelf now under threat – East Antarctica
Chris – Frozen planet 2 – focusing on Arctic ice (BBC iPlayer)
Chat
21:06:20 From Chris Vivian : https://iopscience.iop.org/article/10.1088/1748-9326/ac82ff
21:15:24 From DV Henkel-Wallace : https://archive.ph/OsW9g
21:31:20 From John Nissen : Sea level rise from Antarctica is coming to the fore: https://nypost.com/2022/09/07/antarctica-doomsday-glacier-hanging-on-by-its-fingernails/
21:45:52 From John Nissen : Thwaites could collapse within a few years, producing several feet of SLR.
21:56:04 From Chris Vivian : https://www.science.org/doi/epdf/10.1126/sciadv.abn2465
22:09:31 From Chris Vivian : https://www.nature.com/articles/d41586-022-02168-y#:~:text=But%20over%20the%20past%20ten,pinpoint%20the%20processes%20driving%20it.
22:17:03 From DV Henkel-Wallace : Have to drop off — good call this week.
22:26:25 From Chris Vivian : https://methaneaction.org/
22:27:30 From Chris Vivian : https://www.youtube.com/channel/UCbvK6JuF_tjD9bEBZ1FCfyA - see this recording of the methane action conference |  (Click HERE to view the transcrript) (Click HERE to view the transcrript) |
|
|
|
|
|
| | # |
Name
(N/A = Not Available) |
Title |
Ref Date |
Date Added |
|---|
|
|
|
| Description | This is the second article in a two-part series. Read the first part here.
Climate model scenarios similar to current policies project 2°C of warming before 2050; if James Hansen is right (see Part 1) and warming sharply accelerates, it could be a decade sooner. These outcomes will be driven by the high energy imbalance, continuing high emissions, the accelerating accumulation of heat in the oceans, and decreases in short-term aerosol cooling. |
| Contents | Several years ago a group of eminent scientists proposed a “carbon law”, which said that keeping warming to 2°C required emissions to be halved every decade from 2020 onwards, including a halving between 2020 and 2030, plus some carbon drawdown. Instead, the level of greenhouse gases and coal use both hit record highs in 2023. And the largest national fossil fuel producers plan to keep on expanding production As a result, current government plans worldwide will likely result in emissions in 2050 almost as high as they are today, according to the UN Environment Programme’s 2023 Production Gap report.
Other analyses are broadly consistent:
The International Energy Agency finds that stated national policies will result in oil and gas production in 2050 as high as 2020; with coal halved.
The OECD finds that a world economy four times larger than today is projected to need 80% more energy in 2050; and without new policy action and the global energy mix in 2050 will not differ significantly from today.
The intentions of the world’s five largest fossil fuel producers are clear — and civilisation-threatening — as reported by the UN:
In China, oil production is projected to be flat to 2050, but gas will increase more than 60 percent from 2020 to 2050, while coal use will remain high till 2030 then decline sharply.
In the United States, oil production will grow and then remain at record levels to 2050, and gas is projected to continuously and significantly increase to 2050; whilst coal will drop by half.
Projections for Russia are available only to 2035, with coal and gas production projected to increase significantly, while oil remains flat.
In Saudi Arabia, oil production is projected to grow by 26 to 47 percent by 2050, with gas up 40 percent between 2019 and 2050. Together they make up half of the Saudi economy.
And in Australia, one of the world’s top two liquified natural gas and coal exporters, gas production is projected to stay above the current level for the next 15 years, with coal remaining high over the same period, above 450 million metric tons annually.
We are heading towards 3–4°C.
This outlook suggests Earth is heading towards 3°C of warming and perhaps a good deal more, because current climate models which project warming of around 2.7°C do not adequately account for all the system-level reinforcing feedbacks.
In 2021, the pre-eminent UK international affairs think-tank Chatham House said a “plausible worst-case scenario” is 3.5°C or more, which could be an underestimate if tipping points are reached sooner than the orthodox science suggests. This now seems to be the reality.
A clear majority of scientists expected warming of more than 3°C, and 82% expected to see catastrophic impacts of climate change in their lifetime, according to a 2021 survey by the journal Nature.
Questions about the size of the aerosol forcing, and the related issue of how sensitive the climate is to changes in greenhouse gases, remain an issue of scientific contention.
New climate history research published in December 2023, based on a study of the last 66 million years, concluded that global temperature may be more sensitive to CO2 levels than current models estimate. It showed that the last time CO2 levels were as high as today was around 14 million year ago, which is longer than previous estimates, and that climate sensitivity — the amount of warming resulting from a doubling of atmospheric CO2 — may be between between 5°C and 8°C, compared to the IPCC orthodoxy of 1.5–4.5°C.
The level of greenhouse gases is currently around 560 parts per million, double the pre-industrial level. Some of those gases such as methane are short-lived so this level of forcing is not written in stone, but nevertheless if Hansen et al. are right that a doubling may lead to around 4–5°C of warming, then another 30 years of high emissions means humans will have created an increasingly unliveable planet.
Has the impact of aerosols been widely understood? In what the New York Times described as “an eye-opening Nature commentary”, Geeta Persad and her colleagues wrote in late 2022 that “overall, vast emissions of aerosols since the start of the industrial age have had a profound cooling effect” and that without them “the global warming we see today would be 30 to 50 percent greater”, warning that “the impacts of aerosols on climate risk are often ignored”.
In 2018, a group of eminent scientists explored the potential — once warming had exceeded the 1.5–2°C range — for self-reinforcing positive feedbacks in major elements of the climate system to push passed a planetary threshold that would prevent temperature stabilisation, and drive the system to a “Hothouse Earth”. They warned that “we are in a climate emergency… this is an existential threat to civilisation”.
The 2023 State of the Climate Report: Entering uncharted territory warned of: “potential collapse of natural and socioeconomic systems in such a world [of 2.6°C warming] where we will face unbearable heat, frequent extreme weather events, food and fresh water shortages, rising seas, more emerging diseases, and increased social unrest and geopolitical conflict.”
Whatever the words, the understanding is widely shared that contemporary nations and societies, and likely the global social system, are heading towards collapse. “If we carry on the way we are going now, I can’t see this civilisation lasting to the end of this century”, says Professor Tim Lenton. The US Defence Secretary Lloyd Austin III calls the risks “existential”.
Opening the Innovation Zero Congress in London in May 2023, Potsdam Institute Director Prof. Johan Rockstrom described the path we are on:
“2.5°C global mean surface temperature rise is a disaster. It’s something that humanity has absolutely no evidence that we can cope with… [There] would be a 10-metre sea-level rise. There would be a collapse of all the big biomes on planet Earth – the rainforest, many of the temperate forests – abrupt thawing of permafrost, we will have complete collapse of marine biology… Over one-third of the planet around the equatorial regions will be uninhabitable because you will pass the threshold of health, which is around 30°C. It’s only in some parts of the Sahara Desert today that has that kind of average temperature.”
Chatham House’s Climate Risk Assessment 2021 concludes that by 2050 global food demand would be 50% higher, but crop yields may drop by 30%. As desertification spreads across the dry sub-tropics, and one-third of the planet experiences unprecedented heat, it is not difficult to see why they concluded that cascading climate impacts will “drive political instability and greater national insecurity, and fuel regional and international conflict”.
What is worse is the setback to climate action posed by current conflicts and military posturing in Europe, the Middle East and east Asia, which are huge political distractions from dealing with the greatest threat to humanity, and all of which have the potential to spread more widely.
To maintain military flexibility, the US insisted in 1997 that direct military carbon emissions be excluded from international carbon accounting. Those emissions, around 5 percent of the total global, are far less than the indirect emissions from conflict, as recent estimates here and here indicate.
Projections show that by 2100 the expansion of the Sahara due to desertification will embrace Israel/Palestine, as well as spreading across the Mediterranean into Spain, Italy, Greece and Turkey (see map).
The Australian Prime Minister has finally spoken out about the escalating climate threat whilst inspecting damage from the recent Queensland floods: “All of this is a reminder that the science told us that climate change would mean there would be more extreme weather events and they would be more intense. And unfortunately, we are seeing that play out with the number of events that we’re having to deal with right around Australia”.
Just so, except that in common with leaders globally, the Australian government continues to have its head stuck in the sand about the real risks climate change now represents. It refuses to release an intelligence assessment of climate-security risks, and has fumbled a domestic climate risk assessment.
As a result, the community remains ill-informed and unprepared for what is coming. |
|
|
|
|
| Author | David Spratt and Ian Dunlop |
| Description | For climate change, 2023 was an “unprecedented” year, “absolutely gobsmackingly bananas” and “scary” and “frightening”. And that was what climate scientists said! The UN Secretary General called it the year in which humanity crossed into a new climate era — an age of “global boiling”.
Climate disruption shocked climate scientists in 2023. “Surprising. Astounding. Staggering. Unnerving. Bewildering. Flabbergasting. Disquieting. Gobsmacking. Shocking. Mind boggling,” said Prof. Ed Hawkins when September 2023 exceeded the previous September record by a huge 0.5°C.
The decline in Antarctic sea-ice extent was much greater than model projections, leading the National Snow and Ice Data Centre’s Walt Meier to exclaim: “It’s so far outside anything we’ve seen, it’s almost mind- blowing.”
Many records were set for new climate extremes — record heat, rainfall and floods — with some of it driven by the destabilisation of the polar jet stream. “We are hitting record breaking extremes much sooner than I expected. That’s frightening, scary, and concerning, and it really suggests that we’re not as aware of what’s coming as we thought we were,” said Sarah Perkins-Kirkpatrick of the University of NSW. |
| Contents | With devastating extreme heat and storms and floods, 2023 was the first year 1.5°C warmer than the 1850-1900 baseline, and both Antarctic sea-ice loss and record northern hemisphere sea-surface temperatures were way beyond the ranges projected by climate models.
Datasets of global temperatures vary a little depending on method, but two of the most significant are Berkeley Earth which put 2023 at 1.54°C above the pre-industrial (1850-1900) level, and Copernicus/ECMWF at 1.48°C.
Berkeley said that “a single year exceeding 1.5°C is a stark warning sign of how close the overall climate system has come to exceeding this Paris Agreement goal. With greenhouse gas emissions continuing to set record highs, it is likely that climate will regularly exceed 1.5°C in the next decade.”
2023 was notable for:
Global average warming hitting the 1.5°C mark, and new monthly records for global temperature every month from June to December. The October to December period was 1.74°C.
New national record high annual averages for an estimated 77 countries.
The first year that global average ocean surface temperatures exceeded 1°C, with once-in-a-century levels of warmth in the North Atlantic.
Two days in November when global average temperature, for the first time ever, reached 2°C above the pre-industrial levels.
Catastrophic flooding from Greece to Beijing to Vermont, and earlier in the year major flooding in New Zealand associated with a rain bomb and then cyclone Gabrielle.
Severe wildfires in Europe, Russia, Maui and North America; fires in Canada burned 18.5 million hectares of land.
The 2023 extremes were a shock. Prof. Katharine Hayhoe told the Guardian that: “We have strongly suspected for a while that our projections are underestimating extremes, a suspicion that recent extremes have proven likely to be true… We are truly in uncharted territory in terms of the history of human civilisation on this planet.”
Explanations for 2023 are incomplete, but warming is accelerating and 2024 is likely to be hotter
What happened in 2023 was not what scientists’ models anticipated at the beginning of the year and fell well outside the confidence intervals of any of the estimates. Carbon Brief says that “while there are a number of factors that researchers have proposed to explain 2023’s exceptional warmth, scientists still lack a clear explanation for why global temperatures were so unexpectedly high… researchers are just starting to disentangle the causes of the unexpected extreme global heat the world experienced in 2023”.
One person who has a clear view is the former NASA climate chief James Hansen who says that “the 1.5 degree limit is deader than a doornail” and warns that warming will accelerate to 1.7°C by 2030 and “2°C will be reached by the late 2030s”. |
|
|
|
|
Agenda
Clive "“ Simplified graphics making case for MCB
Sev "“ Project updates from Rebecca, John, Bru, Clive
John N "“ Tipping points
Chat
20:07:40 From Chris Vivian - GESAMP WG 41 : Global heating will pass 1.5C threshold this year, top ex-Nasa scientist says https://www.theguardian.com/environment/2024/jan/08/global-temperature-over-1-5-c-climate-change?CMP=Share_iOSApp_Other
20:13:45 From Ursula Head : Happy New Year, everyone!
20:14:41 From Rebecca Bishop - Gadigal lands : https://www.abc.net.au/listen/programs/futuretense/who-are-the-beneficiaries-of-climate-change-/102960772?utm_campaign=abc_listen\u0026utm_content=link\u0026utm_medium=content_shared\u0026utm_source=abc_listen
20:34:18 From Bru Pearce : https://emagazine.com/energy-imbalance/
20:44:47 From Rebecca Bishop - Gadigal lands : Rebrighten.org
20:49:42 From Bru Pearce : https://www.gcsp.ch/Equity4Humanity
20:59:26 From Shaun Fitzgerald : Sorry i arrived v late, and alas i can't stay tonight. But good to see you. Best wishes, Shaun
21:03:18 From Bru Pearce : www.envisionation.org
21:03:51 From Bru Pearce : http://icesfoundation.org/Pages/Home.aspx
21:31:31 From John Macdonald : Apologies, I need to leave now.
Happy to answer any questions on the inventions I mentioned. |  (Click HERE to view the transcrript) (Click HERE to view the transcrript) |
|
|
|
|
| Description | Earth is a cool place
Earth is home to nearly 9 million species of plants and animals, 8 billion humans and surely the most beautiful and diverse nature in the known universe. We can be proud of our incredible planet.
Our planet is in need
Earth is under threat. Global temperatures have risen by around 1.2 degrees since the industrial revolution; the consequences of this warming are already dramatic, but they are minor in comparison to what lies ahead. |
| Contents | The Planet Earth Coolkit
It’s time to act in order to keep the planet cool. Humans can help Earth turn down its thermostat, enhancing its own natural processes to reduce global temperatures. We have grouped these processes together into what we call The Planet Earth Coolkit.
We need you to help us get the message out that global cooling is possible!
Global warming is the biggest threat to mankind. It is time now to Cool Planet Earth. |
|
|
|
|
| Description | Global heating of the Earth system is unequivocal. However, detecting an acceleration of Earth heating has remained elusive to date, despite suggestive evidence of a potential increase in heating rates. In this study, we demonstrate that since 1960, the warming of the world ocean has accelerated at a relatively consistent pace of 0.15 ± 0.05 (W/m2)/decade, while the land, cryosphere, and atmosphere have exhibited an accelerated pace of 0.013 ± 0.003 (W/m2)/decade. This has led to a substantial increase in ocean warming, with a magnitude of 0.91 ± 0.80 W/m2 between the decades 1960-1970 and 2010-2020, which overlies substantial decadal-scale variability in ocean warming of up to 0.6 W/m2. Our findings withstand a wide range of sensitivity analyses and are consistent across different observation-based datasets. The long-term acceleration of Earth warming aligns qualitatively with the rise in CO2 concentrations and the decline in aerosol concentration during the same period, but further investigations are necessary to properly attribute these changes. |
| Contents | In the past 150 years, Earth's climate has been warming at a rate that is unprecedented in at least the last 2000 years1. This human-caused warming has caused widespread adverse impacts and related losses and damages to nature and people, which will continue in the future as global climate continues to warm2. Detecting changes in the rate of warming is crucial for informed decision-making in international climate negotiations, with the aim of limiting global warming to specific levels. However, it remains a significant challenge to detect such changes due to the substantial internal variability of the climate system on a decadal scale (e.g., ref. 3). In this paper, we address this challenge by examining the global heat accumulation rate across the entire climate system, including the ocean, atmosphere, cryosphere, and land. By focusing on this integrated view, rather than solely relying on changes in global mean surface temperature, we can mitigate the impact of variability and gain a more comprehensive understanding4,5. Global heat accumulation in the climate system, resulting from the current positive Earth's Energy Imbalance (EEI) at the top of the atmosphere, is primarily dominated by changes in Global Ocean Heat Content (GOHC)4. GOHC changes account for approximately 90% of the total heat increase in the past fifty years, while land heating, ice melting, and atmospheric warming contribute around 5%, 3%, and 1% respectively6,7,8. Several studies have indicated an increase in the global heat accumulation rate in recent decades, with values rising from 0.50 [0.32 to 0.69] W/m2 during the period 1971-2006 to 0.79 [0.52 to 1.06] W/m2 (90% confidence interval) for the period 2006-2018 (ref.4,6,7,8,9,10,11,12,13,14,15,16,17,18,19,20,21,22 and Fig. 1). Some studies have even suggested a potential doubling of EEI in the last decade compared to the previous one6,17. Figure 1 |
|
|
|
|
|
| | # |
Name
(N/A = Not Available) |
Title |
Ref Date |
Date Added |
|---|
|
|
|
| Description | This is the second article in a two-part series. Read the first part here.
Climate model scenarios similar to current policies project 2°C of warming before 2050; if James Hansen is right (see Part 1) and warming sharply accelerates, it could be a decade sooner. These outcomes will be driven by the high energy imbalance, continuing high emissions, the accelerating accumulation of heat in the oceans, and decreases in short-term aerosol cooling. |
| Contents | Several years ago a group of eminent scientists proposed a “carbon law”, which said that keeping warming to 2°C required emissions to be halved every decade from 2020 onwards, including a halving between 2020 and 2030, plus some carbon drawdown. Instead, the level of greenhouse gases and coal use both hit record highs in 2023. And the largest national fossil fuel producers plan to keep on expanding production As a result, current government plans worldwide will likely result in emissions in 2050 almost as high as they are today, according to the UN Environment Programme’s 2023 Production Gap report.
Other analyses are broadly consistent:
The International Energy Agency finds that stated national policies will result in oil and gas production in 2050 as high as 2020; with coal halved.
The OECD finds that a world economy four times larger than today is projected to need 80% more energy in 2050; and without new policy action and the global energy mix in 2050 will not differ significantly from today.
The intentions of the world’s five largest fossil fuel producers are clear — and civilisation-threatening — as reported by the UN:
In China, oil production is projected to be flat to 2050, but gas will increase more than 60 percent from 2020 to 2050, while coal use will remain high till 2030 then decline sharply.
In the United States, oil production will grow and then remain at record levels to 2050, and gas is projected to continuously and significantly increase to 2050; whilst coal will drop by half.
Projections for Russia are available only to 2035, with coal and gas production projected to increase significantly, while oil remains flat.
In Saudi Arabia, oil production is projected to grow by 26 to 47 percent by 2050, with gas up 40 percent between 2019 and 2050. Together they make up half of the Saudi economy.
And in Australia, one of the world’s top two liquified natural gas and coal exporters, gas production is projected to stay above the current level for the next 15 years, with coal remaining high over the same period, above 450 million metric tons annually.
We are heading towards 3–4°C.
This outlook suggests Earth is heading towards 3°C of warming and perhaps a good deal more, because current climate models which project warming of around 2.7°C do not adequately account for all the system-level reinforcing feedbacks.
In 2021, the pre-eminent UK international affairs think-tank Chatham House said a “plausible worst-case scenario” is 3.5°C or more, which could be an underestimate if tipping points are reached sooner than the orthodox science suggests. This now seems to be the reality.
A clear majority of scientists expected warming of more than 3°C, and 82% expected to see catastrophic impacts of climate change in their lifetime, according to a 2021 survey by the journal Nature.
Questions about the size of the aerosol forcing, and the related issue of how sensitive the climate is to changes in greenhouse gases, remain an issue of scientific contention.
New climate history research published in December 2023, based on a study of the last 66 million years, concluded that global temperature may be more sensitive to CO2 levels than current models estimate. It showed that the last time CO2 levels were as high as today was around 14 million year ago, which is longer than previous estimates, and that climate sensitivity — the amount of warming resulting from a doubling of atmospheric CO2 — may be between between 5°C and 8°C, compared to the IPCC orthodoxy of 1.5–4.5°C.
The level of greenhouse gases is currently around 560 parts per million, double the pre-industrial level. Some of those gases such as methane are short-lived so this level of forcing is not written in stone, but nevertheless if Hansen et al. are right that a doubling may lead to around 4–5°C of warming, then another 30 years of high emissions means humans will have created an increasingly unliveable planet.
Has the impact of aerosols been widely understood? In what the New York Times described as “an eye-opening Nature commentary”, Geeta Persad and her colleagues wrote in late 2022 that “overall, vast emissions of aerosols since the start of the industrial age have had a profound cooling effect” and that without them “the global warming we see today would be 30 to 50 percent greater”, warning that “the impacts of aerosols on climate risk are often ignored”.
In 2018, a group of eminent scientists explored the potential — once warming had exceeded the 1.5–2°C range — for self-reinforcing positive feedbacks in major elements of the climate system to push passed a planetary threshold that would prevent temperature stabilisation, and drive the system to a “Hothouse Earth”. They warned that “we are in a climate emergency… this is an existential threat to civilisation”.
The 2023 State of the Climate Report: Entering uncharted territory warned of: “potential collapse of natural and socioeconomic systems in such a world [of 2.6°C warming] where we will face unbearable heat, frequent extreme weather events, food and fresh water shortages, rising seas, more emerging diseases, and increased social unrest and geopolitical conflict.”
Whatever the words, the understanding is widely shared that contemporary nations and societies, and likely the global social system, are heading towards collapse. “If we carry on the way we are going now, I can’t see this civilisation lasting to the end of this century”, says Professor Tim Lenton. The US Defence Secretary Lloyd Austin III calls the risks “existential”.
Opening the Innovation Zero Congress in London in May 2023, Potsdam Institute Director Prof. Johan Rockstrom described the path we are on:
“2.5°C global mean surface temperature rise is a disaster. It’s something that humanity has absolutely no evidence that we can cope with… [There] would be a 10-metre sea-level rise. There would be a collapse of all the big biomes on planet Earth – the rainforest, many of the temperate forests – abrupt thawing of permafrost, we will have complete collapse of marine biology… Over one-third of the planet around the equatorial regions will be uninhabitable because you will pass the threshold of health, which is around 30°C. It’s only in some parts of the Sahara Desert today that has that kind of average temperature.”
Chatham House’s Climate Risk Assessment 2021 concludes that by 2050 global food demand would be 50% higher, but crop yields may drop by 30%. As desertification spreads across the dry sub-tropics, and one-third of the planet experiences unprecedented heat, it is not difficult to see why they concluded that cascading climate impacts will “drive political instability and greater national insecurity, and fuel regional and international conflict”.
What is worse is the setback to climate action posed by current conflicts and military posturing in Europe, the Middle East and east Asia, which are huge political distractions from dealing with the greatest threat to humanity, and all of which have the potential to spread more widely.
To maintain military flexibility, the US insisted in 1997 that direct military carbon emissions be excluded from international carbon accounting. Those emissions, around 5 percent of the total global, are far less than the indirect emissions from conflict, as recent estimates here and here indicate.
Projections show that by 2100 the expansion of the Sahara due to desertification will embrace Israel/Palestine, as well as spreading across the Mediterranean into Spain, Italy, Greece and Turkey (see map).
The Australian Prime Minister has finally spoken out about the escalating climate threat whilst inspecting damage from the recent Queensland floods: “All of this is a reminder that the science told us that climate change would mean there would be more extreme weather events and they would be more intense. And unfortunately, we are seeing that play out with the number of events that we’re having to deal with right around Australia”.
Just so, except that in common with leaders globally, the Australian government continues to have its head stuck in the sand about the real risks climate change now represents. It refuses to release an intelligence assessment of climate-security risks, and has fumbled a domestic climate risk assessment.
As a result, the community remains ill-informed and unprepared for what is coming. |
|
|
|
|
Agenda
Clive "“ Simplified graphics making case for MCB
Sev "“ Project updates from Rebecca, John, Bru, Clive
John N "“ Tipping points
Chat
20:07:40 From Chris Vivian - GESAMP WG 41 : Global heating will pass 1.5C threshold this year, top ex-Nasa scientist says https://www.theguardian.com/environment/2024/jan/08/global-temperature-over-1-5-c-climate-change?CMP=Share_iOSApp_Other
20:13:45 From Ursula Head : Happy New Year, everyone!
20:14:41 From Rebecca Bishop - Gadigal lands : https://www.abc.net.au/listen/programs/futuretense/who-are-the-beneficiaries-of-climate-change-/102960772?utm_campaign=abc_listen\u0026utm_content=link\u0026utm_medium=content_shared\u0026utm_source=abc_listen
20:34:18 From Bru Pearce : https://emagazine.com/energy-imbalance/
20:44:47 From Rebecca Bishop - Gadigal lands : Rebrighten.org
20:49:42 From Bru Pearce : https://www.gcsp.ch/Equity4Humanity
20:59:26 From Shaun Fitzgerald : Sorry i arrived v late, and alas i can't stay tonight. But good to see you. Best wishes, Shaun
21:03:18 From Bru Pearce : www.envisionation.org
21:03:51 From Bru Pearce : http://icesfoundation.org/Pages/Home.aspx
21:31:31 From John Macdonald : Apologies, I need to leave now.
Happy to answer any questions on the inventions I mentioned. |  (Click HERE to view the transcrript) (Click HERE to view the transcrript) |
|
|
|
|
| Author | Michael MacCracken, Suzanne Reed |
| Description | Official reports on the state of the climate and progress toward meeting the 2015 Paris Agreement 1.5°C and 2.0°C targets for limiting global warming set the stage for COP28 opening on November 30. The findings were alarming to many, but apparently, not alarming enough for negotiators gathered in Dubai to accept reality. Bottom line, the temperature is rising at an accelerating pace and progress toward meeting the Paris targets is sorely lagging. It may not be as bad as it could have been if we did nothing, but that is a poor excuse for COP28 refusing to admit that the current global climate strategy is an epic fail. |
| Contents | Central to the continuing failure of the COP process is the sole reliance on reducing greenhouse gas (GHG) emissions and removing accumulated emissions from the atmosphere to limit temperature rise. A much broader approach is urgently needed to restore and stabilize climate conditions that can sustain all life forms. It is time to integrate climate cooling into a credible global strategy.
Climate change and the severity of its impacts are increasing much faster than calculated by various models and analyses projecting future conditions. What’s more, the impacts are interacting to push critical ecological, hydrologic, and glacial systems over thresholds, known as tipping points, thus amplifying climate disasters. This truth is being told by current events, not speculative assumptions. The reports released to inform COP28 deliberations confirm 2023 as the worst year yet of climate change with the Global South and vulnerable populations around the world bearing the brunt. |
|
|
|
| 4 |
rKPBHoPyXas (1:21) |
NOAC meeting- 13th Nov 2023 - MCB, Tipping points, Open letter to IMO, Cooling credits, Website demo |
11/13/23 |
1/28/24 |
|
|
|
| 4 |
rKPBHoPyXas (1:21) |
NOAC meeting- 13th Nov 2023 - MCB, Tipping points, Open letter to IMO, Cooling credits, Website demo |
11/13/23 |
1/28/24 |
|
Agenda
Robert T "“ Marine Cloud brightening
"“ Tipping points
Ron B "“ Open letter to IMO "“ please sign it, and spread the word!
John M "“ Cooling credit market: e.g. aerosols, sea surface, etc
Bruce P ¬"“ NOAC website demo
Chris V "“ NREL "“ Renewable energy to power marine CDR
"“ COP 28 "“ Virtual Ocean Pavillion
Chat
20:07:06 From Chris Vivian - GESAMP WG 41 : Mission Analysis for Marine Renewable
Energy To Provide Power for Marine
Carbon Dioxide Removal - https://www.nrel.gov/docs/fy23osti/87165.pdf
20:09:32 From Chris Vivian - GESAMP WG 41 : COP28 Virtula Ocean Pavilion - https://cop28oceanpavilion.vfairs.com/
20:14:30 From Robert Tulip : https://rebrighten.org/
20:23:02 From Rebecca Bishop - Gadigal lands : Professor Symes said: "As an electrochemist, I"™ve spent the last 15 years developing sustainable fuels and chemical processes in the drive towards net zero. Now, as an ARIA Programme Director I want to explore technologies for actively reducing atmospheric carbon dioxide levels and for climate intervention at the regional and global scale.
"Limiting further increases in atmospheric carbon dioxide levels by achieving net zero is necessary but insufficient to prevent the worst consequences of climate change. The full effects of delaying action will be felt by our children and grandchildren. We need to know what our options are for responsible climate intervention technologies, and if, where and when we should deploy them. If we are serious about combating climate change, we need to evaluate these technologies now."
20:23:17 From Rebecca Bishop - Gadigal lands : https://www.gla.ac.uk/news/headline_1001798_en.html
20:27:20 From Doug Grandt : Financial Times notification this morning: Bit.ly/GoreFT25Oct23 "¦ inspired me to post on Facebook "In nearly a year, Al Gore hasn"™t got Dr. Hansen"™s message"”still spewing I.P.C.C. \u0026 Dr. Mann-think.
😵"💫🙄😳🤔ðŸ¤ðŸ«£ðŸ¤—😑🤮
(heads up at 6:18"“7:00)
20:32:08 From Jonathan Cole : What is it? What is distracting everyone, what do they really concentrate on?
20:40:44 From Jonathan Cole : Perhaps the subject, global temperature, is in the same category of concern to individuals as |  (Click HERE to view the transcrript) (Click HERE to view the transcrript) |
|
|
|
|
Agenda
John N – BBC News says 1.5C will arrive twice as soon as previously thought.
- Letter to Guardian re Hansen Global warming in pipeline paper
Chris V – Report from Chatham House – focusing on 1.5 is flawed, focus on tipping points
Hugh H – MCB and controlling hurricanes
Chris V – Compendium of interventions – in the chat
Sev – NOAC website progress – integration of Blue Cooling Initiative, HPAC ,PRAG
Ron – Comments on bunker fuel letter?
Clive – Just Have A Think – recent video very good.
– Radiative forcing to -0.26 W/m2 waterfall chart demo.
Chat
20:06:23 From Chris Vivian - GESAMP WG 41 : Carbon emissions threaten 1.5C climate threshold sooner than thought – report - https://www.bbc.co.uk/news/science-environment-67242386
20:07:16 From Chris Vivian - GESAMP WG 41 : Global warming: Why focusing on 1.5C is flawed - https://www.chathamhouse.org/publications/the-world-today/2023-10/global-warming-why-focusing-15c-flawed?utm_source=Chatham%20House&utm_medium=email&utm_campaign=14169358_CH%20-%20Content%20Newsletter%20-%2017.10.23&utm_content=Global-Title&dm_i=1S3M,8FP5A,NODY6,YTVON,1
20:10:08 From Chris Vivian - GESAMP WG 41 : FROZEN ARCTIC: Compendium of interventions to slow down, halt, and reverse the effects of climate change in the Arctic and northern regions - https://new.uarctic.org/media/to0bjpal/frozen-arctic-rra-compendium.pdf
20:10:48 From Ron Baiman : First part of Sharpe's book is very good on risk analysis and climate.
20:12:43 From Ron Baiman : Bunker fuel letter link: https://docs.google.com/document/d/1WNsRI8GbyZgdso39ptKuKFI2HA6ZuBni/edit?usp=sharing&ouid=116465941111195452408&rtpof=true'sd=true
20:13:23 From Herb Simmens : The Nov 16th HPAC meeting will feature Mike McCracken discussing the differing approaches to addressing risk that various professions and institutions take
20:15:28 From Herb Simmens : This Thursday Nov 2 HPAC meeting at 3:30 PM EDT 19:30 PM GMT will feature Doug MacMartin of Cornell on SRM/SAI..
20:17:00 From Robert Chris : https://www.nature.com/articles/s41558-023-01848-5
20:19:42 From Herb Simmens : This blog post has a link to the paper in the first sentence https://scienceisshiny.wordpress.com/2023/10/30/carbon-budgets-how-hard-is-the-paris-agreement-now/
20:21:37 From Bill Chapman, Brooklyn : I was horrified when Trump and some Republicans started talking about |  (Click HERE to view the transcrript) (Click HERE to view the transcrript) |
|
|
|
|
|
|
|
|
| | # |
Name
(N/A = Not Available) |
Title |
Ref Date |
Date Added |
|---|
|
|
|
| Description | There is clear scientific consensus that carbon
dioxide removal (CDR) — alongside a strong
prioritization of greenhouse gas emissions
reduction — will be required at an immense,
multi-gigatonne (Gt) annual scale by mid-century
to limit warming to 1.5 or even 2°C.1
Covering 71%
of the planet’s surface, the ocean has served as a
critically important sink for anthropogenic carbon
dioxide (CO2), absorbing over 25% of annual
emissions.2
The ocean has also absorbed about
90% of the heat that has accumulated in the Earth
system due to rising atmospheric CO2.
3
The ocean
thus provides a vital climate mitigation function,
but this has come at significant, and increasing,
cost to ocean health, marine ecosystems, and
biodiversity |
| Contents | Given its enormous scale, the ocean has an
outsized potential role to play in advancing CDR
to the level required to meet Paris Agreement
targets. Marine CDR (mCDR) has the potential,
when responsibly deployed and scaled, to
offer significant climate benefits, while also
contributing to sustainable economic development
for coastal communities, maritime nations, and
small island developing states (SIDS). Additionally,
certain mCDR approaches may yield co-benefits
such as improvements to ocean health, via local
mitigation of ocean acidification, to coastal
ecosystems and commercial aquaculture.
Examples of mCDR include:
Ocean alkalinity enhancement (OAE) via
electrochemical systems, or the physical
application of clean alkaline minerals to
coastlines, coastal watersheds, or the open ocean;
Electrochemical or photochemical systems that
directly remove CO2 from the ocean;
Dedicated cultivation or harvesting of aquatic
biomass, including macroalgae and microalgae (for
sinking to the deep ocean, long-duration terrestrial
storage, or potential incorporation into long-lived
products); and
Restoration, enhancement, and scaling of carbon
sinks associated with seagrass, mangroves, and
other coastal marine ecosystems (coastal “blue
carbon”). |
|
|
|
|
Agenda
Bruce P - NOAC Website
Chris V "“ Marine CDR research projects announced.
Brian VH "“ Jim Hansen"™s Oct note "“ El Nino Fizzles, Earth Sizzles
Stephen S "“ what would doubling of aerosol do?
Clive E "“ What would satellite aerosol measurement do?
Methane PNAS conf "“ Copenhagen papers.
Chat
21:07:22 From Chris Vivian - GESAMP WG 41 : US Announcements on marine CDR:\rWhite House Forms Committee On Marine Carbon Removal - https://carbonherald.com/white-house-forms-committee-on-marine-carbon-removal/\rMarine Carbon Dioxide Removal: Potential Ways to Harness the Ocean to Mitigate Climate Change - https://www.whitehouse.gov/ostp/news-updates/2023/10/06/marine-carbon-dioxide-removal-potential-ways-to-harness-the-ocean-to-mitigate-climate-change/ \rMarine Carbon Dioxide Removal Fast Track Action Committee - https://www.noaa.gov/sites/default/files/2023-10/mCDR_FTAC_charter_2023_09_19_approved.pdf\rAnnouncing $24.3m Investment Advancing Marine Carbon Dioxide Removal Research - https://oceanacidification.noaa.gov/focus_areas/carbon-dioxide-removal/\r21:08:50 From Chris Vivian - GESAMP WG 41 : Hansen paper https://www.columbia.edu/~jeh1/mailings/2023/ElNinoFizzles.13October2023.pdf\r21:44:09 From Clive Elsworth : https://seao2-cdr.eu/\r21:47:15 From Clive Elsworth : http://www.gesamp.org/site/assets/files/1723/ocean_climate_intervention_projects_sept_2023.xlsx\r22:05:07 From baiman"™s iPhone : Yes!! Especially if aviation can emit enough aerosol to have a significant cooling impact/. Apologies I"™m with grandson so can"™t participate in person !\r22:11:20 From Hugh Hunt : https://www.ukri.org/opportunity/jet-zero-aviations-non-co2-impacts-on-the-climate/?utm_medium=email\u0026utm_source=govdelivery\r22:11:31 From Hugh Hunt : That's the JetZero link\r22:13:37 From Hugh Hunt : Maybe this one can be used for Oceans:\r\rhttps://www.ukri.org/opportunity/pushing-the-frontiers-of-environmental-research-jan-2024/\r22:21:52 From baiman"™s iPhone : Thank you Hugh\r22:31:33 From John Macdonald : Also there"™s lthe Methane Tracker 2020 |  (Click HERE to view the transcrript) (Click HERE to view the transcrript) |
|
|
|
|
Agenda
John M - Ocean abundance is problematic?
Clive "“ ocean acidification?
Stephen "“ Energy for MCB vs Energy reflected
"“ Explain insolation diagram
John M - Atmospheric river over Australia, Warm weather in Europe
Sev "“ Who is going to COP27? Hopes?
Stephen "“ SAI conference in Colorado |  (Click HERE to view the transcrript) (Click HERE to view the transcrript) |
|
|
|
|
- We discussed Sev Clarke"™s new idea of measuring largescale ocean CDR by using sonar to measure krill populations.
- Robert Tulip commented on the recent The Hill article on ocean CDR.
- Towards the end we discussed the importance of ocean carbonate chemistry for determining long term sequestration and pH in the ocean. Brian pointed out the ocean"™s natural "˜antacid medicine"™ against acidification: huge carbonate deposits that cover the seafloor and on seamounts down to the carbonate compensation depth.
Brian also mentioned Eddy Covariance, which is explained here: https://en.wikipedia.org/wiki/Eddy_covariance |  (Click HERE to view the transcrript) (Click HERE to view the transcrript) |
|
|
|
| 5 |
nature.com/articles/s4159... |
An earth system model shows self-sustained thawing of permafrost even if all man-made GHG emissions stop in 2020 |
11/12/20 |
11/8/23 |
|
|
|
| 5 |
nature.com/articles/s4159... |
An earth system model shows self-sustained thawing of permafrost even if all man-made GHG emissions stop in 2020 |
11/12/20 |
11/8/23 |
|
| Description | The risk of points-of-no-return, which, once surpassed lock the world into new dynamics, have been discussed for decades. Recently, there have been warnings that some of these tipping points are coming closer and are too dangerous to be disregarded. In this paper we report that in the ESCIMO climate model the world is already past a point-of-no-return for global warming. In ESCIMO we observe self-sustained thawing of the permafrost for hundreds of years, even if global society stops all emissions of man-made GHGs immediately. We encourage other model builders to explore our discovery in their (bigger) models, and report on their findings. The thawing (in ESCIMO) is the result of a continuing self-sustained rise in the global temperature. This warming is the combined effect of three physical processes: (1) declining surface albedo (driven by melting of the Arctic ice cover), (2) increasing amounts of water vapour in the atmosphere (driven by higher temperatures), and (3) changes in the concentrations of the GHG in the atmosphere (driven by the absorption of CO2 in biomass and oceans, and emission of carbon (CH4 and CO2) from thawing permafrost). This self-sustained, in the sense of no further GHG emissions, thawing process (in ESCIMO) is a causally determined, physical process that evolves over time. It starts with the man-made warming up to the 1950s, leading to a rise in the amount of water vapour in the atmosphere--further lifting the temperature, causing increasing release of carbon from thawing permafrost, and simultaneously a decline in the surface albedo as the ice and snow covers melts. To stop the self-sustained warming in ESCIMO, enormous amounts of CO2 have to be extracted from the atmosphere. |
| Contents | The possibility of points-of-no-return in the climate system has been discussed for two decades1,2,3. A point-of-no-return can be seen as a threshold which, once surpassed, fundamentally changes the dynamics of the climate system. For example, by triggering irreversible processes like thawing of the permafrost, drying of the rainforests, or acidification of surface waters. Recently, Lenton et al.4 summarized the global situation and warned that thresholds may be closer in time than commonly believed. The purpose of this article is to report that we have identified a point-of-no-return in our climate model ESCIMO - and that it is already behind us. ESCIMO is a "reduced complexity earth system" climate model5 which we run from 1850 to 2500. In ESCIMO the global temperature keeps rising to 2500 and beyond, irrespective of how fast humanity cuts the emissions of man-made greenhouse gas (GHG) emissions. The reason is a cycle of self-sustained thawing of the permafrost (caused by methane release), lower surface albedo (caused by melting ice and snow) and higher atmospheric humidity (caused by higher temperatures). This cycle appears to be triggered by global warming of a mere?+?0.5 °C above the pre-industrial level. |
|
|
|
|
|
| | # |
Name
(N/A = Not Available) |
Title |
Ref Date |
Date Added |
|---|
|
|
|
| Author | David Spratt and Ian Dunlop |
| Description | For climate change, 2023 was an “unprecedented” year, “absolutely gobsmackingly bananas” and “scary” and “frightening”. And that was what climate scientists said! The UN Secretary General called it the year in which humanity crossed into a new climate era — an age of “global boiling”.
Climate disruption shocked climate scientists in 2023. “Surprising. Astounding. Staggering. Unnerving. Bewildering. Flabbergasting. Disquieting. Gobsmacking. Shocking. Mind boggling,” said Prof. Ed Hawkins when September 2023 exceeded the previous September record by a huge 0.5°C.
The decline in Antarctic sea-ice extent was much greater than model projections, leading the National Snow and Ice Data Centre’s Walt Meier to exclaim: “It’s so far outside anything we’ve seen, it’s almost mind- blowing.”
Many records were set for new climate extremes — record heat, rainfall and floods — with some of it driven by the destabilisation of the polar jet stream. “We are hitting record breaking extremes much sooner than I expected. That’s frightening, scary, and concerning, and it really suggests that we’re not as aware of what’s coming as we thought we were,” said Sarah Perkins-Kirkpatrick of the University of NSW. |
| Contents | With devastating extreme heat and storms and floods, 2023 was the first year 1.5°C warmer than the 1850-1900 baseline, and both Antarctic sea-ice loss and record northern hemisphere sea-surface temperatures were way beyond the ranges projected by climate models.
Datasets of global temperatures vary a little depending on method, but two of the most significant are Berkeley Earth which put 2023 at 1.54°C above the pre-industrial (1850-1900) level, and Copernicus/ECMWF at 1.48°C.
Berkeley said that “a single year exceeding 1.5°C is a stark warning sign of how close the overall climate system has come to exceeding this Paris Agreement goal. With greenhouse gas emissions continuing to set record highs, it is likely that climate will regularly exceed 1.5°C in the next decade.”
2023 was notable for:
Global average warming hitting the 1.5°C mark, and new monthly records for global temperature every month from June to December. The October to December period was 1.74°C.
New national record high annual averages for an estimated 77 countries.
The first year that global average ocean surface temperatures exceeded 1°C, with once-in-a-century levels of warmth in the North Atlantic.
Two days in November when global average temperature, for the first time ever, reached 2°C above the pre-industrial levels.
Catastrophic flooding from Greece to Beijing to Vermont, and earlier in the year major flooding in New Zealand associated with a rain bomb and then cyclone Gabrielle.
Severe wildfires in Europe, Russia, Maui and North America; fires in Canada burned 18.5 million hectares of land.
The 2023 extremes were a shock. Prof. Katharine Hayhoe told the Guardian that: “We have strongly suspected for a while that our projections are underestimating extremes, a suspicion that recent extremes have proven likely to be true… We are truly in uncharted territory in terms of the history of human civilisation on this planet.”
Explanations for 2023 are incomplete, but warming is accelerating and 2024 is likely to be hotter
What happened in 2023 was not what scientists’ models anticipated at the beginning of the year and fell well outside the confidence intervals of any of the estimates. Carbon Brief says that “while there are a number of factors that researchers have proposed to explain 2023’s exceptional warmth, scientists still lack a clear explanation for why global temperatures were so unexpectedly high… researchers are just starting to disentangle the causes of the unexpected extreme global heat the world experienced in 2023”.
One person who has a clear view is the former NASA climate chief James Hansen who says that “the 1.5 degree limit is deader than a doornail” and warns that warming will accelerate to 1.7°C by 2030 and “2°C will be reached by the late 2030s”. |
|
|
|
|
| Description | The intensifying impacts of climate change are exceeding projections and amplifying the risk of catastrophic harm to the environment and society throughout the 21st century. Planned and proposed rates of emissions reduction and removal are not proceed |
| Contents | The intensifying impacts of climate change are exceeding projections and amplifying the risk of catastrophic harm to the environment and society throughout the 21st century. Planned and proposed rates of emissions reduction and removal are not proceeding at a pace or magnitude to meet either the 1.5°C or 2.0°C targets of the Paris Agreement. Moreover, the impacts, damage and loss occurring at today's 1.2°C of global warming are already significantly disrupting the environment and society. Relying exclusively on greenhouse gas (GHG) emissions reduction and removal without including climate cooling options is thus proving incompatible with responsible planetary stewardship. Multiple approaches to exerting a cooling influence have the potential to contribute to offset at least some of the projected climate disruption if deployed in the near term. Employed thoughtfully, such approaches could be used to limit global warming to well below 1° C, a level that has led to large reductions in sea ice, destabilization of ice sheets, loss of biodiversity, and transformation of ecosystems. An effective plan for avoiding |
|
|
|
|
|
| | # |
Name
(N/A = Not Available) |
Title |
Ref Date |
Date Added |
|---|
|
|
|
| Description | Extreme weather is 'smacking us in the face' with worse to come, but a 'tiny window' of hope remains, say leading climate scientists |
| Contents | Extreme weather is 'smacking us in the face' with worse to come, but a 'tiny window' of hope remains, say leading climate scientists |
|
|
|
| 2 |
rebrighten.org/ |
Reflecting Sunlight to Cool the Ocean Surface and Reduce Global Warming |
11/1/23 |
11/14/23 |
|
|
|
| 2 |
rebrighten.org/ |
Reflecting Sunlight to Cool the Ocean Surface and Reduce Global Warming |
11/1/23 |
11/14/23 |
|
| Description | Rebrighten.org is a not for profit organization newly established to study, promote and deploy Marine Cloud Brightening (MCB) as the most effective, safe and rapid available method to reverse global warming. We seek to raise USD $5 million to implement our proposal to prove the feasibility of MCB as a way to cool and re-brighten the planet, alongside broader existing efforts to mitigate warming by cutting and removing greenhouse gases. Help us create a brighter, safer future for the planet & humanity by supporting this crucial project. |
| Contents | What is Marine Cloud Brightening and Why is it necessary?
Marine Cloud Brightening (MCB) is potentially the first feasible way to start cooling the planet by reflecting more sunlight back to space.
Deployment would help slow global temperature rise, refreeze the poles and mitigate extreme weather such as storms, fires, droughts, heatwaves and floods.
Sea salt sprayed into the lower atmosphere in targeted areas is expected to prove a harmless way to reverse warming.
MCB has potential to reverse sea level rise with benefit to cost ratio estimated at 50,000 to 1.
MCB has been recognised for 50 years as a simple way to cool the oceans. It relies on the ‘Twomey Effect’, from its discoverer Dr Sean Twomey, who showed that clouds with smaller drops reflect more sunlight and are brighter than clouds with larger drops. Dr John Latham then showed that the optimal cloud drop size for MCB can be produced with sea salt particles smaller than one micron. |
|
|
|
| 3 |
kQkyouPOrD4 (15) |
The heat may not kill you, but the global food crisis might! |
9/17/23 |
10/13/23 |
|
|
|
| 3 |
kQkyouPOrD4 (15) |
The heat may not kill you, but the global food crisis might! |
9/17/23 |
10/13/23 |
|
A super strong El Niño and record high global sea surface temperatures are set to deliver devastating extreme weather events all over the planet in 2024. They will be extremely costly and traumatic for many millions of people around the world. But the real concern is what the consequences of those events will show us about the fragility and vulnerability our global food supply network is. A worrying portent of our near-term future?
Help support this channels independence at
http://www.patreon.com/justhaveathink
Or with a donation via Paypal by clicking here
https://www.paypal.com/cgi-bin/webscr?cmd=_s-xclick&hosted_button_id=GWR73EHXGJMAE'source=url
You can also help keep my brain ticking over during the long hours of research and editing via the nice folks at BuyMeACoffee.com
https://www.buymeacoffee.com/justhaveathink
Video Transcripts available at our website
http://www.justhaveathink.com
Research Links
MINTEC - El Niño prediction for 2023 24
https://www.mintecglobal.com/top-stories/global-el-ni%C3%B1o-forecasts-predict-a-very-strong-event-for-2023/24#:~:text=August%20ENSO%20(El%20Ni%C3%B1o-Southern,and%20potentially%20destructive%20weather%20globally.
IPCC 6th Assessment
https://www.ipcc.ch/report/ar6/wg1/chapter/chapter-9/
2023 US billion dollar disasters
https://laist.com/news/climate-environment/2023-has-already-broken-the-us-record-for-billion-dollar-climate-disasters
Ocean Heat Absorption
https://www.theguardian.com/environment/2023/may/15/oceans-have-been-absorbing-the-worlds-extra-heat-but-theres-a-huge-payback#:~:text='The%20ocean%20captures%20more%20than,the%20direct%20energy%20from%20sunlight.
https://www.resilience.org/stories/2023-07-21/ocean-heat-is-off-the-charts-heres-what-that-means-for-humans-and-ecosystems-around-the-world/
Cheng et al 2022 - Ocean Heating
https://link.springer.com/article/10.1007/s00376-022-1461-3
Bentley et al 2022 - Global wheat supplies
https://www.nature.com/articles/s43016-022-00559-y
Hedlund et al 2022 - Impacts of climate change on global food trade networks
https://iopscience.iop.org/article/10.1088/1748-9326/aca68b?gclid=Cj0KCQjwmICoBhDxARIsABXkXlJzc2AvEtmB1-x8MtP1xKd2kNdNDZeitBCGYz_M-ep_7qvhHCK0khYaAsxtEALw_wcB
Callahan et al 2023 - Persistent effects of El Niño on global food supplies
https://www.science.org/doi/10.1126/science.adf2983
Barclays - Global food systems under mounting pressure
https://www.cib.barclays/our-insights/global-food-systems-under-mounting-pressure.html?cid=paidsearch-textads_google_google_themes_food-security_uk-we_food-security_838686827459&gclid=Cj0KCQjwmICoBhDxARIsABXkXlJWhuabO00YyTi3o2vUQVe9NE_eYYLXBnDozmY84pL8IiJjWR7UyfsaAhqCEALw_wcB&gclsrc=aw.ds
https://www.cib.barclays/content/dam/barclaysmicrosites/ibpublic/documents/our-insights/food-security/ImpactSeries_10_brochure_final.pdf
NOAA ENSO Update April 2023
https://www.climate.gov/news-features/blogs/enso/april-2023-enso-update-el-nino-watch?page=1
WMO reports
https://public.wmo.int/en/media/press-release/earth-had-hottest-three-month-period-record-unprecedented-sea-surface
https://public.wmo.int/en/media/press-release/world-meteorological-organization-declares-onset-of-el-ni%C3%B1o-conditions
Other media reports on El Niño and food supply network
https://edition.cnn.com/2023/06/29/economy/el-nino-economic-impact/index.html
https://edition.cnn.com/2023/07/05/world/wmo-el-nino-warmer-weather-climate-intl-hnk/index.html
https://edition.cnn.com/2023/05/17/world/global-warming-breach-wmo-climate-intl/index.html
Check out other YouTube Climate Communicators
zentouro: https://www.youtube.com/user/zentouro
Climate Adam: https://www.youtube.com/user/ClimateAdam
Kurtis Baute: https://www.youtube.com/user/ScopeofScience
Levi Hildebrand: https://www.youtube.com/user/The100LH
Simon Clark: https://www.youtube.com/user/SimonOxfPhys
Sarah Karvner: https://www.youtu |  (Click HERE to view the transcrript) (Click HERE to view the transcrript) |
|
|
|
|
NOAC Meeting points - 11th July 2022
The whole transcript plus meeting notes is here:
https://docs.google.com/document/d/1yVnVuCvfALGi8nT0QG3qEjiurN-eZRug5DFEzikDZkc/edit?usp=sharing
Robert: There is a false orthodoxy that nothing can be done to avoid 1.5 C. Albedo enhancement appears to be taboo or doesn’t exist. There is no serious policy engagement. Climate complications (like medical complications) might be avoided with SRM. It’s ignored because of the moral hazard argument that demonises the fossil fuel (FF) industry. The only thing that could now prevent biodiversity loss, sea level rise (SLR), extreme weather events etc is to increase albedo. Saying otherwise has no scientific basis.
[Albedo enhancement also means Solar Radiation Management – SRM, e.g. Stratospheric Aerosol Injection - SAI, Marine Cloud Brightening – MCB]
Aria: Climate denial to avoid the necessary technology changes is pretty evil. It has already caused millions of deaths.
Robert: Committed warming was in place even during the 1990s.
Aria: It’s important not to oppose reducing emissions.
Robert: Cutting emissions is marginal to stabilising the climate. Too much focus on emissions reduction detracts from what would have more effect. Even greenhouse gas removal (GGR) should not be the primary response.
Aria: We need an ‘all of the above’ approach, and not be adversarial. People should continue operating in their area of expertise. We want to build alliances and not risk masking underlying problems.
Brian: The public need to be brought on board step-by-step that decarbonization is necessary but not sufficient, and planetary albedo needs to be restored. Eg Arctic albedo has reduced from 0.8 to 0.2.
The fossil fuel industry lied for decades, but today they’re not all the same. E.g. Shell has committed to reducing scope 3 (customer) emissions by 45% by 2035. FF and mining companies are among the few with the cash to scale huge technology transformations. That should continue, with maybe 10% of resources going to cooling interventions to avoid the worst effects of warming this century as we get back to a healthy CO2 level, which will take longer.
Robert: Yes, decarbonisation investment should continue, but the priority should move to albedo restoration to avoid the current big risk of Arctic collapse. The public is unaware of the inadequacy of decarbonization.
Brian: Albedo contains many components, but we can say: “Make the planet brighter – and smarter.â€
Aria: Yes, I love that framing.
Sev: Brightening is much less painful than emissions reduction. Priorities need reordering: 1. Brightening, 2. GGR, 3. Emissions reduction, 4. Thermal management, (e.g. cirrus cloud thinning), 5. Adaptation - sea walls are only temporarily effective.
Me: Polarisation is less problematic than demonization. (Prof Kotkin)
Robert: Fossil fuels have delivered high standards of living. It’s risky to reduce those. Demonization hampers civilised dialogue.
Sev: You catch more flies with honey than vinegar.
Aria: But pollution is damaging to human health. Energy efficiency would ameliorate that.
Brian: We could be at the start of a microbial methane bomb. An atmospheric methane depletion intervention could cut the warming rate in half. Perhaps focus 80% of efforts on that, and rebrightening.
Robert: Increasing Arctic albedo is the best way to slow permafrost methane emissions and curb the accelerating feedback loops.
Ron: I’m trying to compile a document of cooling technologies. 1.2 billion people live in countries that depend on fossil fuel earnings, and the Republican Party has been bought by the FF industry.
Bru: Large environmental groups have insufficient understanding of the problems. Closing down the global economy would cause untold suffering. Planetary brightening is an absolute necessity, as is reducing emissions as fast as we can. Our job is to educate the public, while kee |  (Click HERE to view the transcrript) (Click HERE to view the transcrript) |
|
|
|
|
Main discussions:
Peter Carter’s recent video warning of today's radiative forcing of over 3 W/m2 spelling climate disaster (see chat link) seems at odds with the figure of 1.7 W/m2 from Prof Julia Slingo (recently retired director of the Hadley centre). 1.7 also aligns more closely with the NASA GISS graph of net forcing after anthropogenic aerosols are taken into account (scroll down to the first graph): https://data.giss.nasa.gov/modelforce/ Steven pointed out (once again) that 1.7 W/m2 is only ½% of the energy arriving from the sun, of 340 W/m2 . This again makes a compelling case for cooling by marine cloud brightening (MCB).
I probably confused many by writing on the agenda “Atmospheric acidity causes more methaneâ€. I meant “more methane depletion†i.e. naturally occurring iron salt aerosol depletes methane more efficiently when the pH is low (between pH 0.5 and 2 ). The loss of SO2 from ships and coal power stations may therefore be leading to higher ocean aerosol pHs, and therefore less atmospheric methane depletion.
Brian pointed out that determining whether methane lifetime is increasing might be done by measuring 14CH4 concentration, because 14C is an unstable carbon isotope produced only in the upper atmosphere. But I've since seen that 14C comes from nitrogen. Perhaps let's discuss next time.
Brian also said a newly increased source of methane seems to be mainly microbial methane, determined by isotopic signature (see chat link below).
We discussed possible use of artificial Iron Salt Aerosol to double up to provide MCB in addition to methane depletion. The rationale would be that it also makes white clouds, and if its technology will already be there, then why not follow the plan Steven promotes of emitting it in the right places at the right times to curb extreme weather events, as well as cooling the oceans?
There were more technical discussions of droplet size, size distribution and Cl atom production from sun irradiated droplets (especially from the 1 hr point).
Carbonyl sulphide (COS) has been proposed as a cooling intervention for the upper atmosphere, but is rather poisonous, and its production of an SO2 based aerosol in the right places at the right time seems haphazard.
Other points:
John N and Ron B asked us to review their temperature trajectory and HPAC documents, that make the case for cooling.
David HW foresaw the Supreme Court decision that was then announced mid last week, crippling the EPA's ability to direct emission reductions. He also pointed out (right at the start) that governments are not set up to address emissions effectively.
Both Brian and David HW said there's no such thing as social licence, just lots of people who say “noâ€. The best way to gain general acceptance is not through doom and gloom [or finger wagging, CE], but from positive messages.
Chat Links
From Clive Elsworth : Peter Carter’s video on radiative forcing: https://www.youtube.com/watch?v=-3-SBXrdjw0
From Dr Brian von Herzen : https://research.noaa.gov/article/ArtMID/587/ArticleID/2769/New-analysis-shows-microbial-sources-fueling-rise-of-atmospheric-methane
From Mannajo Greene : Climate activists elected officials and others need more education on how the global climate system work. |  (Click HERE to view the transcrript) (Click HERE to view the transcrript) |
|
|
|
|
|
| | # |
Name
(N/A = Not Available) |
Title |
Ref Date |
Date Added |
|---|
|
|
|
| Author | David Spratt and Ian Dunlop |
| Description | For climate change, 2023 was an “unprecedented” year, “absolutely gobsmackingly bananas” and “scary” and “frightening”. And that was what climate scientists said! The UN Secretary General called it the year in which humanity crossed into a new climate era — an age of “global boiling”.
Climate disruption shocked climate scientists in 2023. “Surprising. Astounding. Staggering. Unnerving. Bewildering. Flabbergasting. Disquieting. Gobsmacking. Shocking. Mind boggling,” said Prof. Ed Hawkins when September 2023 exceeded the previous September record by a huge 0.5°C.
The decline in Antarctic sea-ice extent was much greater than model projections, leading the National Snow and Ice Data Centre’s Walt Meier to exclaim: “It’s so far outside anything we’ve seen, it’s almost mind- blowing.”
Many records were set for new climate extremes — record heat, rainfall and floods — with some of it driven by the destabilisation of the polar jet stream. “We are hitting record breaking extremes much sooner than I expected. That’s frightening, scary, and concerning, and it really suggests that we’re not as aware of what’s coming as we thought we were,” said Sarah Perkins-Kirkpatrick of the University of NSW. |
| Contents | With devastating extreme heat and storms and floods, 2023 was the first year 1.5°C warmer than the 1850-1900 baseline, and both Antarctic sea-ice loss and record northern hemisphere sea-surface temperatures were way beyond the ranges projected by climate models.
Datasets of global temperatures vary a little depending on method, but two of the most significant are Berkeley Earth which put 2023 at 1.54°C above the pre-industrial (1850-1900) level, and Copernicus/ECMWF at 1.48°C.
Berkeley said that “a single year exceeding 1.5°C is a stark warning sign of how close the overall climate system has come to exceeding this Paris Agreement goal. With greenhouse gas emissions continuing to set record highs, it is likely that climate will regularly exceed 1.5°C in the next decade.”
2023 was notable for:
Global average warming hitting the 1.5°C mark, and new monthly records for global temperature every month from June to December. The October to December period was 1.74°C.
New national record high annual averages for an estimated 77 countries.
The first year that global average ocean surface temperatures exceeded 1°C, with once-in-a-century levels of warmth in the North Atlantic.
Two days in November when global average temperature, for the first time ever, reached 2°C above the pre-industrial levels.
Catastrophic flooding from Greece to Beijing to Vermont, and earlier in the year major flooding in New Zealand associated with a rain bomb and then cyclone Gabrielle.
Severe wildfires in Europe, Russia, Maui and North America; fires in Canada burned 18.5 million hectares of land.
The 2023 extremes were a shock. Prof. Katharine Hayhoe told the Guardian that: “We have strongly suspected for a while that our projections are underestimating extremes, a suspicion that recent extremes have proven likely to be true… We are truly in uncharted territory in terms of the history of human civilisation on this planet.”
Explanations for 2023 are incomplete, but warming is accelerating and 2024 is likely to be hotter
What happened in 2023 was not what scientists’ models anticipated at the beginning of the year and fell well outside the confidence intervals of any of the estimates. Carbon Brief says that “while there are a number of factors that researchers have proposed to explain 2023’s exceptional warmth, scientists still lack a clear explanation for why global temperatures were so unexpectedly high… researchers are just starting to disentangle the causes of the unexpected extreme global heat the world experienced in 2023”.
One person who has a clear view is the former NASA climate chief James Hansen who says that “the 1.5 degree limit is deader than a doornail” and warns that warming will accelerate to 1.7°C by 2030 and “2°C will be reached by the late 2030s”. |
|
|
|
| 2 |
Word |
Climate crisis “out of control” (Letter sent to the Guardian containing SAI proposal but not published) |
10/10/23 |
11/26/23 |
|
|
|
| 2 |
Word |
Climate crisis “out of control” (Letter sent to the Guardian containing SAI proposal but not published) |
10/10/23 |
11/26/23 |
|
| Author | John Nissen (On behalf of the Planetary Restoration Action Group (PRAG)) |
| Description | We have a historically unprecedented climate emergency. The planet is now heating so fast that the Paris-agreed ceiling of 1.5°C global mean temperature is liable to be reached next year and 2°C within a decade or two. This rapid heating will be boosted by Arctic meltdown: with less sunshine reflected by snow and ice, and with the release of the potent greenhouse gas, methane, from thawing permafrost. Arctic meltdown also affects the jet stream: we can expect the double-whammy of increased global heating and stuck jet stream patterns to produce ever more extreme heatwaves, droughts and wildfires. The climate crisis is indeed spiralling “out of control” (the Guardian headline on 6th October). Our only chance of seizing control, before catastrophe becomes inevitable, is through rapid, emergency cooling intervention. We owe it to the young people of today that we grasp the nettle and prepare for solar geoengineering: injecting sulphur dioxide into the stratosphere to mimic the cooling action of major volcanic eruptions. Experimental injection could even start next year: injection anywhere between 45N and 65N would safely limit the lifetime of the aerosol produced to a few months according to climate models. The risks from rapid full-scale intervention could prove tiny in comparison with the risk of leaving intervention too late to prevent catastrophic climate change for much of the world. To boot, a variety of appropriate interventions, together with a drastic cut in greenhouse gas emissions, could restore the planet to a safe, sustainable, biodiverse and productive state within the lifetimes of our young people. |
|
|
|
|
| Author | Franz Oeste, Clive Elsworth, |
| Description | Climate catalyst is a nature-based method that can be used to provide powerful cooling interventions. It employs the same substances and chemistry that have operated in nature for millions of years, especially during the glacial periods, to bring about the same cooling effects. All of its processes operate in the troposphere and biosphere, albeit they benefit the stratosphere, enabling it to recover a healthy ozone layer. We regard it as a potentially important mechanism for delaying or even reversing the onset of tipping points such as catastrophic polar and mountain ice sheet collapse, and the collapse of the Atlantic Meridional Overturning Circulation (AMOC). |
| Contents | Climate catalyst is a nature-based method that can be used to provide powerful cooling interventions. It employs the same substances and chemistry that have operated in nature for millions of years, especially during the glacial periods, to bring about the same cooling effects. All of its processes operate in the troposphere and biosphere, albeit they benefit the stratosphere, enabling it to recover a healthy ozone layer. We regard it as a potentially important mechanism for delaying or even reversing the onset of tipping points such as catastrophic polar and mountain ice sheet collapse, and the collapse of the Atlantic Meridional Overturning Circulation (AMOC).
Climate catalyst works in two main ways. Firstly, it speeds up the natural removal from the air of powerful greenhouse gases such as methane. Secondly, it increases the direct cooling influence of clouds, by brightening them and making them last longer in the sky. Clouds are brightened the same way as proposed for Marine Cloud brightening (MCB). The difference is that instead of using evaporated seawater droplets to increase cloud droplet number, microscopic particles of a non-toxic substance perform the same function. When the particles rainout they 'flocculate' (a process used in wastewater treatment, to purify water), removing any potential nanoparticle hazard. They then gradually transform to clay mineral, which is completely inert.
We suggest that an additional safe cooling technology such as climate catalyst should be made available, because it offers additional advantages over MCB. For example, in addition to cooling large areas of ocean and ice sheets, climate catalyst could keep areas at risk of wildfire moist before the fire season. When wildfires do occur, it could reduce the climate warming effect of the smoke by shortening its lifetime in the air. That is because climate catalyst speeds up the natural process by which the smoke particles rain out. It also lightens their colour, which reduces their warming influence in several ways. In addition, climate catalyst helps to protect the ozone layer by preventing smoke particles and other ozone destroyers from entering the stratosphere.
Methane and smoke (black carbon aerosol) are very powerful greenhouse warming agents. If Climate Catalyst proves able to remove them from the air safely and cost-effectively, they represent the 'low-hanging fruit' of climate action. The additional benefit of cloud cooling makes Climate Catalyst an even more compelling proposition.
Existing industry processes and low-cost substances can be used to produce climate catalyst particles. It could be dispersed in remote areas from ships, drones, and land-based dispersal facilities. The bulk of the climate cooling needed could be achieved by dispersing it from existing ships traversing the ocean. Climate catalyst utilizes some of the components of ships' emission gases for its enhanced methane removal chemistry.
Currently Climate Catalyst is a proposal looking for funding to be tested, first in laboratories to develop the safest, most effective formulation, then field trialled to optimize cooling operations. If the trials are successful, we would request regulatory support from governments to ensure it is used in well-coordinated, peaceful, environmentally beneficial ways. Only then should it be scaled up to cool the world's oceans and ice sheets. We accept that even that should be done incrementally, to ensure the effects are always beneficial. Fortunately, if Climate Catalyst needs to be stopped for any reason, it gets rained out of the air within a few days to weeks. At that point it no longer has any direct effect on the climate.
While active in the air Climate catalyst might alter weather patterns, therefore dispersal operations need careful planning. It should be dispersed when/where it is needed in a way that induces favourable weather across the globe on a long-term basis. Such a program would doubtless be controversial and would need to be managed transparently by a trusted intergovernmental body of experienced meteorologists and climate scientists.
Humanity needs to come to terms with the fact that we have profoundly altered the Earth's atmosphere and climate. Therefore, climate cooling interventions will be needed for the foreseeable future until the atmosphere's gas concentrations can be restored to their preindustrial levels. |
|
|
|
|
| Description | Carbon emissions need to fall to zero by 2050 but can the world achieve that goal? |
| Contents | As wildfires tear across southern Europe the need for urgent action on climate change becomes ever clearer. Reducing carbon emissions is a global challenge but can we meet it? |
|
|
|
|
|
|
|
|
| | # |
Name
(N/A = Not Available) |
Title |
Ref Date |
Date Added |
|---|
|
|
|
Sev presented his ideas on boosting methanotrophs in shallow seas, melting tundra and other places.
We also discussed the potentially large carbon sequestration carried out by krill and other Diel Vertically Migrating creatures. If true it is completely unrecognized, and looks to deserve further urgent research, and likely curbed fishing.
Brian spoke of two recent impressive books: Regenesis by George Monbiot (on sustainable farming), and All we can Save, narrated by Jane Fonda (on the stress experienced by climate activists).
We talked about our own stress, and how we remain motivated in the face of huge ignorance of the general public on the issues and potentially strong solutions. The solutions we discuss are generally of both technical and human based. Grant mentioned the power of grandchildren in focusing their grandparents on the big problems coming down the line.
It was a great meeting. Thanks, all who were able to attend.
This time I hosted from a hotel room, which may have made it difficult for some to connect. Apologies if that was the case. I expect to be home again for the next NOAC meeting.
Clive |  (Click HERE to view the transcrript) (Click HERE to view the transcrript) |
|
|
|
|
| Description | A Practical, Get-Your-Hands-in-the-Soil Manual Global climate change, increasing pollution, and continued rapid population growth is wreaking havoc on the planet. Stabilizing the environment at safe levels requires a large-scale restoration of damaged ecosystems. Geotherapy: Innovative Methods of Soil Fertility Restoration, Carbon Sequestration, and Reversing CO2 Increase outlines the basic concepts of geotherapy and highlights the importance of healing the biosphere's ability to store soil ca |
| Contents | Global climate change, increasing pollution, and continued rapid population growth is wreaking havoc on the planet. Stabilizing the environment at safe levels requires a large-scale restoration of damaged ecosystems. Geotherapy: Innovative Methods of Soil Fertility Restoration, Carbon Sequestration, and Reversing CO2 Increase outlines the basic concepts of geotherapy and highlights the importance of healing the biosphere's ability to store soil carbon to prevent climate change impacts. Facing challenges head on, it addresses how and why policymakers have underestimated the long-term impacts of climate change and how we can correct the flawed carbon management mechanisms today. The book also factors in where carbon can be most effectively stored, how quickly that can be done, and the practical and policy actions needed to get there. |
|
|
|
|
|
| | # |
Name
(N/A = Not Available) |
Title |
Ref Date |
Date Added |
|---|
|
|
|
| Description | The ocean regulates our climate and can significantly buffer the worst impacts of climate change, absorbing human-induced carbon dioxide emissions and |
| Contents | Analysis finds that full implementation of ocean-based climate solutions that are ready for action now could reduce the "emissions gap" by up to 35 percent on a 1.5°C pathway in 2050. |
|
|
|
|
Agenda
Anton - Climate solutions website
Clive "“ High Sea Surface Temperatures
Rebecca / Robert C "“ CCRC MCB progress
Sev "“ Turquoise hydrogen
John M "“ Nutrient Upweller
Chris V "“ Co-removing methane CO2
Chat
21:01:08 From Rebecca Bishop - Gadigal lands : Hello Franz, nice to see you :-)
21:03:16 From Rebecca Bishop - Gadigal lands : This sounds wonderful, thanks Anton.
21:09:37 From mycomputer : https://www.biofuelsdigest.com/bdigest/2023/06/20/a-new-hydrogen-from-methane-via-pyrolysis-without-the-co2-release/
21:10:01 From Chris Vivian - GESAMP WG 41 : https://www.researchsquare.com/article/rs-2959117/v1
21:10:11 From Rebecca Bishop - Gadigal lands : I'm going to turn off my camera as I need to eat breakfast; I'm going to a conference with registration 8-9am. I'll switch it on if I'm going to speak :-)
21:10:51 From Chris Vivian - GESAMP WG 41 : Solar chimney - https://www.sciencedirect.com/science/article/abs/pii/S0038092X23003675
21:32:01 From Rebecca Bishop - Gadigal lands : Thank you for such a clear statement of the questions, Anton, and your statement of |  (Click HERE to view the transcrript) (Click HERE to view the transcrript) |
|
|
|
|
| Description | Nobody really knows how much it would cost to avoid the worst impacts of climate change. Yet historian Yuval Noah Harari's analysis, based on the work of scientists and economists, indicates that humanity might avert catastrophe by investing the equivalent of just two percent of global GDP into climate solutions. He makes the case that preventing ecological cataclysm will not require the major global disruptions many fear and explains that we already have the resources we need -- it's just a matter of shifting our priorities. |
|
|
|
|
|
| | # |
Name
(N/A = Not Available) |
Title |
Ref Date |
Date Added |
|---|
|
|
|
| Description | There is clear scientific consensus that carbon
dioxide removal (CDR) — alongside a strong
prioritization of greenhouse gas emissions
reduction — will be required at an immense,
multi-gigatonne (Gt) annual scale by mid-century
to limit warming to 1.5 or even 2°C.1
Covering 71%
of the planet’s surface, the ocean has served as a
critically important sink for anthropogenic carbon
dioxide (CO2), absorbing over 25% of annual
emissions.2
The ocean has also absorbed about
90% of the heat that has accumulated in the Earth
system due to rising atmospheric CO2.
3
The ocean
thus provides a vital climate mitigation function,
but this has come at significant, and increasing,
cost to ocean health, marine ecosystems, and
biodiversity |
| Contents | Given its enormous scale, the ocean has an
outsized potential role to play in advancing CDR
to the level required to meet Paris Agreement
targets. Marine CDR (mCDR) has the potential,
when responsibly deployed and scaled, to
offer significant climate benefits, while also
contributing to sustainable economic development
for coastal communities, maritime nations, and
small island developing states (SIDS). Additionally,
certain mCDR approaches may yield co-benefits
such as improvements to ocean health, via local
mitigation of ocean acidification, to coastal
ecosystems and commercial aquaculture.
Examples of mCDR include:
Ocean alkalinity enhancement (OAE) via
electrochemical systems, or the physical
application of clean alkaline minerals to
coastlines, coastal watersheds, or the open ocean;
Electrochemical or photochemical systems that
directly remove CO2 from the ocean;
Dedicated cultivation or harvesting of aquatic
biomass, including macroalgae and microalgae (for
sinking to the deep ocean, long-duration terrestrial
storage, or potential incorporation into long-lived
products); and
Restoration, enhancement, and scaling of carbon
sinks associated with seagrass, mangroves, and
other coastal marine ecosystems (coastal “blue
carbon”). |
|
|
|
| 2 |
rebrighten.org/ |
Reflecting Sunlight to Cool the Ocean Surface and Reduce Global Warming |
11/1/23 |
11/14/23 |
|
|
|
| 2 |
rebrighten.org/ |
Reflecting Sunlight to Cool the Ocean Surface and Reduce Global Warming |
11/1/23 |
11/14/23 |
|
| Description | Rebrighten.org is a not for profit organization newly established to study, promote and deploy Marine Cloud Brightening (MCB) as the most effective, safe and rapid available method to reverse global warming. We seek to raise USD $5 million to implement our proposal to prove the feasibility of MCB as a way to cool and re-brighten the planet, alongside broader existing efforts to mitigate warming by cutting and removing greenhouse gases. Help us create a brighter, safer future for the planet & humanity by supporting this crucial project. |
| Contents | What is Marine Cloud Brightening and Why is it necessary?
Marine Cloud Brightening (MCB) is potentially the first feasible way to start cooling the planet by reflecting more sunlight back to space.
Deployment would help slow global temperature rise, refreeze the poles and mitigate extreme weather such as storms, fires, droughts, heatwaves and floods.
Sea salt sprayed into the lower atmosphere in targeted areas is expected to prove a harmless way to reverse warming.
MCB has potential to reverse sea level rise with benefit to cost ratio estimated at 50,000 to 1.
MCB has been recognised for 50 years as a simple way to cool the oceans. It relies on the ‘Twomey Effect’, from its discoverer Dr Sean Twomey, who showed that clouds with smaller drops reflect more sunlight and are brighter than clouds with larger drops. Dr John Latham then showed that the optimal cloud drop size for MCB can be produced with sea salt particles smaller than one micron. |
|
|
|
|
| Author | Peter Fiekowsky and Carole Douglis |
| Description | Costs determine scalability, and costs vary by a factor of 30,000 |
| Contents | One purpose of CDR is to provide “offsets” that legitimize continuing emissions.
The other is to restore the pre-industrial climate by 2050 by removing legacy CO2.
Carbon-dioxide removal (CDR)) tops the tech headlines with increasing frequency, leading to the impression that we are rapidly developing a multitude of mostly industrial options for creating a safe climate.
What many miss is that the vast majority of “carbontech” CDR approaches are designed to develop the carbon-offset market. By definition, offsets only compensate for continued emissions. They do not touch the 1,000 gigatons of legacy CO2 that is causing most of the climate havoc.
In fact, CDR today serves two distinct policy purposes. Each has merit, yet achieving the two goals requires quite different approaches and budgets and would create strikingly different results.
The two goals of CDR are:
Developing a CDR industry that underpins the carbon-offset market—thus adhering literally to the 1992 United Nations goal to “stabilize” greenhouse gas levels. Today this means stabilizing at dangerous levels never before experienced by our species. This is of course now called “net-zero emissions;” and
Following what appears to be the original intent of the United Nations Framework Convention on Climate Change: to restore GHG levels proven safe for humanity and nature as we know it. Restoring historically safe GHG levels is commonly called “climate restoration.”
|
|
|
|
|
| Description | We need a new way of talking about global warming. UN Secretary General António
Guterres underscored this when he said the “era of global boiling” has arrived. Although
we have made remarkable progress on a very complex problem over the past thirty years,
we have a long way to go before we can keep the global temperature increase to below 2°C
relative to the pre-industrial times. Climate models suggest that this next decade is critical
if we are to avert the worst consequences of climate change. The world must continue
to reduce greenhouse gas emissions, and find ways to adapt and build resilience among
vulnerable communities. At the same time, we need to find new ways to remove carbon
dioxide from the atmosphere in order to chart a “net negative” emissions pathway. Given
their large capacity for carbon storage, the oceans must be included in consideration of
our multiple carbon dioxide removal (CDR) options (1).
This report focused on ocean iron fertilization (OIF) for marine CDR. This is by no
means a new scientific endeavor. Several members of ExOIS (Exploring Ocean Iron
Solutions) have been studying this issue for decades, but the emergence of runaway climate
impacts has motivated this group to consider a responsible path forward for marine
CDR. That path needs to ensure that future choices are based upon the best science and
social considerations required to reduce human suffering and counter economic and ecological
losses, while limiting and even reversing the negative impacts that climate change
is already having on the ocean and the rest of the planet.
Prior studies have confirmed that the addition of small amounts of iron in some parts
of the ocean is effective at stimulating phytoplankton growth. Through enhanced photosynthesis,
carbon dioxide can not only be removed from the atmosphere but a fraction
can also be transferred to durable storage in the deep sea. However, prior studies were
not designed to quantify how effective this storage can be, or how wise OIF might be as
a marine CDR approach. |
| Contents | needed to answer critical questions about the potential efficiency and ecological impacts
of marine CDR (http://oceaniron.org). Owing to concerns surrounding the ethics of marine
CDR, ExOIS is organized around a responsible code of conduct that prioritizes activities
for the collective benefit of our planet with an emphasis on open and transparent
studies that include public engagement (2; see inset pg. 3).
Our goal is to establish open-source conventions for implementing OIF for marine
CDR that can be assessed with appropriate monitoring, reporting, and verification
(MRV) protocols, going beyond just carbon accounting, to assess ecological and other
non-carbon environmental effects (eMRV). As urgent as this is, it will still take 5 to 10
years of intensive work and considerable resources to accomplish this goal.
We present here a “Paths Forward’’ report that stems from a week-long workshop held
at the Moss Landing Marine Laboratories in May 2023 that was attended by international
experts spanning atmospheric, oceanographic, and social sciences as well as legal specialists
(see inside back cover). At the workshop, we reviewed prior OIF studies, distilled
the lessons learned, and proposed several paths forward over the next decade to lay the
foundation for evaluating OIF for marine CDR. Our discussion very quickly resulted in
a recommendation for the need to establish multiple “Ocean Iron Observatories’’ where,
through observations and modeling, we would be able to assess with a high degree of
certainty both the durable removal of atmospheric carbon dioxide—which we term the
“centennial tonne”—and the ecological response of the ocean.
3 PATHS FORWARD FOR EXPLORING OCEAN IRON FERTILIZATION
In a five-year phase I period, we prioritize five major research activities:
1. Next generation field studies
Studies of long-term (durable) carbon storage will need to be longer (year or more) and
larger (>10,000 km2) than past experiments, organized around existing tools and models, but
with greater reliance on autonomous platforms. While prior studies suggested that ocean
systems return to ambient conditions once iron infusion is stopped, this needs to be verified.
We suggest that these next field experiments take place in the NE Pacific to assess the
processes controlling carbon removal efficiencies, as well as the intended and unintended
ecological and geochemical consequences.
2. Regional, global and field study modeling
Incorporation of new observations and model intercomparisons are essential to accurately
represent how iron cycling processes regulate OIF effects on marine ecosystems and carbon
sequestration, to support experimental planning for large-scale MRV, and to guide decision
making on marine CDR choices.
3. New forms of iron and delivery mechanisms
Rigorous testing and comparison of new forms of iron and their potential delivery
mechanisms is needed to optimize phytoplankton growth while minimizing the financial
and carbon costs of OIF. Efficiency gains are expected to generate responses closer to those
of natural OIF events.
4. Monitoring, reporting, and verification
Advances in observational technologies and platforms are needed to support the development,
validation, and maintenance of models required for MRV of large-scale OIF deployment. In
addition to tracking carbon storage and efficiency, prioritizing eMRV will be key to developing
regulated carbon markets.
5. Governance and stakeholder engagement
Attention to social dimensions, governance, and stakeholder perceptions will be essential
from the start, with particular emphasis on expanding the diversity of groups engaged in
marine CDR across the globe. This feedback will be a critical component underlying future
decisions about whether to proceed, or not, with OIF for marine CDR.
Paramount in the plan is the need to move carefully. Our goal is to conduct these five activities in parallel
to inform decisions steering the establishment of ocean iron observatories at multiple locations in phase
II. When completed, this decadal plan will provide a rich knowledge base to guide decisions about if,
when, where, and under what conditions OIF might be responsibly implemented for marine CDR.
The consensus of our workshop and this report is that now is the time for actionable studies to begin.
Quite simply, we suggest that some form of marine CDR will be essential to slow down and reverse the
most severe consequences of our disrupted climate. OIF has the potential to be one of these climate
mitigation strategies. We have the opportunity and obligation to invest in the knowledge necessary to
ensure that we can make scientifically and ethically sound decisions for the future of our planet. |
|
|
|
| 5 |
nytimes.com/2023/10/08/op... |
Opinion | Africa Needs Its Debts Paused So It Can Prepare for Climate Catastrophe - The New York Times |
10/8/23 |
11/8/23 |
|
|
|
| 5 |
nytimes.com/2023/10/08/op... |
Opinion | Africa Needs Its Debts Paused So It Can Prepare for Climate Catastrophe - The New York Times |
10/8/23 |
11/8/23 |
|
| Description | Instead of receiving funds to address the climate crisis, African nations are borrowing money to rebuild at a cost up to eight times that of the rich world. |
| Contents | By William Ruto, Moussa Faki Mahamat, Akinwumi Adesina and Patrick Verkooijen |
|
|
|
|
|
| | # |
Name
(N/A = Not Available) |
Title |
Ref Date |
Date Added |
|---|
|
|
|
Agenda
Metta "“ Symposium - citizens assembly, global in scope, for discussing climate repair?
Sev "“ Is global evapotranspiration good?
John M "“ What are the sources of cloud formation?
Chat
20:12:11 From Jonathan Cole : What is a citizens"™ assembly?\rCitizens"™ assemblies are not new, and are gaining popularity around the world. They empower people, communities and entire countries to make important decisions in a fair and deeply democratic way.\r\rThe Citizens"™ Assembly on Climate and Ecological Justice will bring together a "mini public" of everyday people to investigate, discuss and make recommendations on how to respond to the climate emergency. These people will be randomly selected, like a jury, to reflect the whole country in terms of gender, age, ethnicity, education level and geography. They will hear balanced information from experts and those most affected by the emergency and then discuss what they have learned openly and honestly in small groups. Together they will work through their differences before drafting and voting on recommendations. The process is run by non-governmental organisations under independent oversight.\r20:16:35
From Jonathan Cole : ClimateViewer - YouTube\rWebJim is the creator of ClimateViewer Maps on http://climateviewer.org/, where you can monitor our world in real-time on a 3D globe with over 600 unique maps and live\r20:17:44
From Jonathan Cole : I am James Franklin Lee Jr. (Jim Lee) and I help people understand complex ideas by creating maps, timelines, articles, and lectures. I am going to talk about Pollution, Privacy and Propaganda every chance I get because they interest me, affect us, and I care about our Planet. ClimateViewer News is my blog and the central location for all of my research.\r20:25:40
From Jonathan Cole : This Jim Lee seems to be initiating the anti geoengineering push for a statement by the NH state legislature\r20:25:51
From Jonathan Cole : Last month, Republican legislators in New Hampshire introduced a bill that would ban the "intentional release" of chemicals into the air.\r\rThe legislation, called "The Clean Atmosphere Preservation Act," prohibits "the intentional release of polluting emissions, including cloud seeding, weather modification, excessive electromagnetic radio frequency, and microwave radiation and making penalties for violation of such prohibition."\r\rIt also provides penalties for violations.\r20:28:28
From Metta Spencer : Https://tosavetheworld.ca\r20:28:41 From Robert Chris : prohibits "the intentional release of polluting emissions, Doesn't that mea no more fossil fuel emissions?\r20:47:28
From Robert Chris : Kevin Anderson\r20:56:20
From Bruce Parker : NOAC Website https://myccnews.org/noac/\r20:57:46
From Jonathan Cole : Great statement: Choose something non controversial enough to get funding. |  (Click HERE to view the transcrript) (Click HERE to view the transcrript) |
|
|
|
|
| Description | Climatic Change - |
| Contents | "Moral hazard" links geoengineering to mitigation via the fear that either solar geoengineering (solar radiation management, SRM) or carbon dioxide removal (CDR) might crowd out the desire to cut emissions. Fear of this crowding-out effect ranks among the most frequently cited risks of (solar) geoengineering. We here test moral hazard versus its inverse in a large-scale, revealed-preference experiment (n?~?340,000) on Facebook and find little to no support for either outcome. For the most part, talking about SRM or CDR does not motivate our study population to support a large US environmental non-profit's mission, nor does it turn them off relative to baseline climate messaging, except when using extreme messengers and framings. Our results indicate the importance of actors and reasoned narratives of (solar) geoengineering to help guide public discourse. |
|
|
|
|
Healthy Planet Action Coalition
https://www.healthyplanetaction.org/
Title: Model Simulations of Climate Interventions Aiming to Offset Future Warming: Insights and Uncertainties
Speaker: Associate Professor Douglas MacMartin, Cornell University
Talk Overview
Decadal-average global warming is approaching 1.2 C and it is likely that the 1.5 C goal from the Paris Agreement will be passed in the next decade or so. Global warming is now being experienced through the increasing likelihood of severe weather, more intense storms, destabilization of major glacial streams, increasing rate of rise of sea level, and more, all driven by the ongoing emissions of greenhouse gas emissions.
With the present and projected pace of emissions mitigation, global warming is projected to at least double before net-zero emissions are reached up to a few decades after mid-century, with corresponding increased impacts and risks.
With all nations committed to the goal of keeping global warming to no more than 1.5 C and climate intervention becoming the only option for preventing further warming, modeling studies have started looking at climate intervention scenarios that would offset further warming, stabilizing the climate at 1.5C, or restoring back to 1.0C or lower.
Professor MacMartin reports on the status of climate stabilization studies using stratospheric aerosol injection (SAI), providing an overview of what would be involved, including options such as more polar-focused deployments, what the resulting stabilized climate would be like and how long it might take to reach a desired cooling, what the key uncertainties are and how they might compare to the types of consequences that might trigger calls for intervention, and what research is needed to provide the firmer information needed for early rather than late-stage emergency intervention to be considered as a potential policy scenario.
The recording includes a powerpoint presentation followed by question and answer with the audience.
Biography (from https://www.mae.cornell.edu/faculty-directory/douglas-macmartin
Douglas MacMartin is an Associate Professor in the Sibley School of Mechanical and Aerospace Engineering at Cornell University.
His research focuses on Sunlight Reflection Methods (SRM, also known as climate engineering, solar geoengineering, or climate intervention), with the aim of helping to develop the knowledge base necessary to support informed future societal decisions in this challenging and controversial field.
He has published extensively on the subject, and in addition to public and academic presentations has provided briefings to the UN Environment Program and testimony to the US Congress, and he was a member of the US National Academies panel that made recommendations on both research and governance in March 2021.
Doug received his Ph.D. in Aeronautics and Astronautics from MIT in 1992; previous positions include United Technologies Research Center (1994-2000) and the California Institute of Technology (2000-2015). His research is funded by NSF and by the Cornell Atkinson Center for a Sustainable Future. |  (Click HERE to view the transcrript) (Click HERE to view the transcrript) |
|
|
|
|
| Description |
Marine Cloud Brightening
Marine cloud brightening refers to an albedo modification technique that aims to increase the reflectivity, and possibly even the lifetimes, of certain clouds in order to reflect more sunlight back into space and partially offset some of the impacts of climate change. The most common proposal for achieving such a goal is to inject naturally occurring sea salt into cloud updrafts. But a variety of methods are being researched. |
| Contents | For example, even if marine cloud brightening could work, it could affect large scale climate and weather patterns if it were used on a large enough scale, say to achieve a level of “radiative forcing” that would be big enough to offset some of the greatest impacts of climate change. (This is because marine cloud brightening could only be implemented in limited areas, where the right kinds of clouds exist, which is perhaps only 10 percent of the planet’s surface.) There are several key risks that need to be better understood.
Still, the fact that more research is needed is true for all geoengineering techniques. So even though the scientists within our group focus most closely on stratospheric aerosol injection, we believe that MCB research is valuable. |
|
|
|
|
| Description | Stratospheric aerosol injection is a solar radiation management (srm) geoengineering or climate engineering approach that uses tiny reflective particles or aerosols to reflect sunlight into space in order to cool the planet and reverse or stop Global Warming. The approach involves spraying reflective sulfate aerosol particles into the stratosphere with high altitude airplanes, tethered balloons, high-altitude blimps or artillery. |
| Contents | Stratosphere definition
The stratosphere (see NOAA image below) is a layer of the Earth’s atmosphere that ranges between 7 to 31 miles above the ground between the Troposphere and the Mesophere. The stratosphere is an ideal target for atmospheric geoengineering because it is relatively isolated from human populations, is accessible by planes (and other transport/delivery methods), and doesn’t have weather such as rain that would cause aerosol spray particles to fall quickly to the ground |
|
|
|
|
|
|
|
|
| | # |
Name
(N/A = Not Available) |
Title |
Ref Date |
Date Added |
|---|
|
|
|
| Description | |
| Contents | 6. I go through the lessons offered by paleoclimate in my new book Our Fragile Moment: How Lessons from Earth's Past Can Help Us Survive the Climate Crisis, and I come away with very different conclusions about what we collectively learn from the Cenozoic cooling, the Pliocene, and the Holocene. The collective evidence from the paleoclimate record tells us that climate models have the climate sensitivity (how much warming we can expect for a specified increase in carbon dioxide concentrations) about right, at least for the range of warming we are likely facing (less than 3C/5F given policies already in place). Of course, if we keep greenhouse gas concentrations elevated for centuries, there is the potential for greater amounts of warming as longer-term climate responses kick in. So it is important to think about strategies for carbon drawdown down the road. Here I agree with Hansen and co-authors. But in terms of what we can expect in the decades ahead, there is no reason, based on the collective evidence from the paleoclimate record, to expect a climate trajectory substantially different from what current generation (i.e. IPCC) models predict. And there is no reason that we can't prevent dangerous levels of warming through concerted efforts to decarbonize the global economy. The obstacles, at least at present, are political, not physical or even technological. |
|
|
|
|
| Description | Human survival depends on this iconic ecosystem, and only one thing will save it. |
| Contents | Human survival depends on this iconic ecosystem, and only one thing will save it. |
|
|
|
|
| Description | 'Shocking' study finds Amazon rainforest will be unable to sustain itself and transport moisture once 'regime shift' occurs |
| Contents | 'Shocking' study finds Amazon rainforest will be unable to sustain itself and transport moisture once 'regime shift' occurs |
|
|
|
|
| Description | Tipping elements are components of the Earth system which may respond nonlinearly to anthropogenic climate change by transitioning toward substantially different long-term states upon passing key thresholds or “tipping points.” In some cases, such changes could produce additional greenhouse gas emissions or radiative forcing that could compound global warming. Improved understanding of tipping elements is important for predicting future climate risks and their impacts. Here we review mechanisms, predictions, impacts, and knowledge gaps associated with 10 notable Earth system components proposed to be tipping elements. We evaluate which tipping elements are approaching critical thresholds and whether shifts may manifest rapidly or over longer timescales. Some tipping elements have a higher risk of crossing tipping points under middle-of-the-road emissions pathways and will possibly affect major ecosystems, climate patterns, and/or carbon cycling within the 21st century. However, literature assessing different emissions scenarios indicates a strong potential to reduce impacts associated with many tipping elements through climate change mitigation. The studies synthesized in our review suggest most tipping elements do not possess the potential for abrupt future change within years, and some proposed tipping elements may not exhibit tipping behavior, rather responding more predictably and directly to the magnitude of forcing. Nevertheless, uncertainties remain associated with many tipping elements, highlighting an acute need for further research and modeling to better constrain risks. |
| Contents | Plain Language Summary
In recent years, discussions of climate change have shown growing interest in “tipping elements” of the Earth system, also imprecisely referred to as “tipping points.” This refers to Earth system components like the tropical rainforests of Amazonia or the Greenland and Antarctic ice sheets which may exhibit large-scale, long-term changes upon reaching critical global warming, greenhouse gas, or other thresholds. Once such thresholds are passed, some tipping elements could in turn produce additional greenhouse gas emissions or change the Earth's energy balance in ways that moderately reinforce warming. In this review, we summarize the current state of scientific knowledge on 10 systems that some have referred to as potential tipping elements of the climate system. We describe the mechanisms important to each system, highlight the response of these systems to climate change so far, and explain the dynamics of potential future changes that these systems could undergo in response to further climate change. Overall, even considering remaining scientific uncertainties, tipping elements will influence future climate change and may involve major impacts on ecosystems, climate patterns, and the carbon cycle starting later this century. Aggressive efforts to stabilize climate change could significantly reduce such impacts. |
|
|
|
|
|
| | # |
Name
(N/A = Not Available) |
Title |
Ref Date |
Date Added |
|---|
|
|
|
| Author | David Spratt and Ian Dunlop |
| Description | For climate change, 2023 was an “unprecedented” year, “absolutely gobsmackingly bananas” and “scary” and “frightening”. And that was what climate scientists said! The UN Secretary General called it the year in which humanity crossed into a new climate era — an age of “global boiling”.
Climate disruption shocked climate scientists in 2023. “Surprising. Astounding. Staggering. Unnerving. Bewildering. Flabbergasting. Disquieting. Gobsmacking. Shocking. Mind boggling,” said Prof. Ed Hawkins when September 2023 exceeded the previous September record by a huge 0.5°C.
The decline in Antarctic sea-ice extent was much greater than model projections, leading the National Snow and Ice Data Centre’s Walt Meier to exclaim: “It’s so far outside anything we’ve seen, it’s almost mind- blowing.”
Many records were set for new climate extremes — record heat, rainfall and floods — with some of it driven by the destabilisation of the polar jet stream. “We are hitting record breaking extremes much sooner than I expected. That’s frightening, scary, and concerning, and it really suggests that we’re not as aware of what’s coming as we thought we were,” said Sarah Perkins-Kirkpatrick of the University of NSW. |
| Contents | With devastating extreme heat and storms and floods, 2023 was the first year 1.5°C warmer than the 1850-1900 baseline, and both Antarctic sea-ice loss and record northern hemisphere sea-surface temperatures were way beyond the ranges projected by climate models.
Datasets of global temperatures vary a little depending on method, but two of the most significant are Berkeley Earth which put 2023 at 1.54°C above the pre-industrial (1850-1900) level, and Copernicus/ECMWF at 1.48°C.
Berkeley said that “a single year exceeding 1.5°C is a stark warning sign of how close the overall climate system has come to exceeding this Paris Agreement goal. With greenhouse gas emissions continuing to set record highs, it is likely that climate will regularly exceed 1.5°C in the next decade.”
2023 was notable for:
Global average warming hitting the 1.5°C mark, and new monthly records for global temperature every month from June to December. The October to December period was 1.74°C.
New national record high annual averages for an estimated 77 countries.
The first year that global average ocean surface temperatures exceeded 1°C, with once-in-a-century levels of warmth in the North Atlantic.
Two days in November when global average temperature, for the first time ever, reached 2°C above the pre-industrial levels.
Catastrophic flooding from Greece to Beijing to Vermont, and earlier in the year major flooding in New Zealand associated with a rain bomb and then cyclone Gabrielle.
Severe wildfires in Europe, Russia, Maui and North America; fires in Canada burned 18.5 million hectares of land.
The 2023 extremes were a shock. Prof. Katharine Hayhoe told the Guardian that: “We have strongly suspected for a while that our projections are underestimating extremes, a suspicion that recent extremes have proven likely to be true… We are truly in uncharted territory in terms of the history of human civilisation on this planet.”
Explanations for 2023 are incomplete, but warming is accelerating and 2024 is likely to be hotter
What happened in 2023 was not what scientists’ models anticipated at the beginning of the year and fell well outside the confidence intervals of any of the estimates. Carbon Brief says that “while there are a number of factors that researchers have proposed to explain 2023’s exceptional warmth, scientists still lack a clear explanation for why global temperatures were so unexpectedly high… researchers are just starting to disentangle the causes of the unexpected extreme global heat the world experienced in 2023”.
One person who has a clear view is the former NASA climate chief James Hansen who says that “the 1.5 degree limit is deader than a doornail” and warns that warming will accelerate to 1.7°C by 2030 and “2°C will be reached by the late 2030s”. |
|
|
|
|
| Description | Alarmingly, the rate of ocean warming has nearly doubled in the past decade compared to the 1990s, according to the study. |
| Contents | The world's oceans, often referred to as the "climate's heat sink," are now experiencing unprecedented rates of warming. Researchers at the University of New South Wales (UNSW Sydney) have uncovered the acceleration of ocean warming and have pinpointed the areas that are absorbing the most heat. |
|
|
|
|
| Description | West Antarctica is headed for decades of rapid melting no matter how quickly humans cut greenhouse gas emissions, and 2023 shattered records for missing sea ice around the continent. |
| Contents | A trio of new scientific analyses about the loss of ice in Antarctica paint a picture of a continent in trouble. Sea ice is disappearing, gigantic portions of the West Antarctic ice sheet are crumbling and even relatively stable East Antarctica is showing worrying changes. |
|
|
|
|
| Global ocean surface temperatures are so high that climate scientists are describing them as |  (Click HERE to view the transcrript) (Click HERE to view the transcrript) |
|
|
|
|
Agenda
Clive - Climate Catalyst slides, DMS to make more marine clouds? Floating corals?
Ron "“ SO2 loss is warming the oceans? Hausfather doubts it.
Bru "“ The ocean has got greener? In places. Dryden"™s work on ocean surface pollution. Brian "“ changes in ocean temperature affecting phytoplankton population dynamics.
No time to discuss:
John N "“ Reasons for sudden increases in surface temperatures NH and SH, Arctic, Antarctic. Brian "“ Changes in cloud cover,
Bru "“ Fire impacts, Rebecca "“ fire management.
Chat
21:29:34 From Herb Simmens : https://news.exeter.ac.uk/faculty-of-environment-science-and-economy/tipping-points-can-be-triggered-unexpectedly-by-dangerous-rates-of-change/
21:30:27 From Ron Baiman : Thanks Herb! Interesting.
21:31:24 From Bru Pearce : https://www.windy.com/-Clouds-clouds?clouds,15.670,163.799,4,i:pressure
21:43:18 From Ron Baiman : This is Hausfather report claiming that DMS in northern hemisphere shipping lanes largely saturates potential of increasing cloud cover with sulfur aerosols: https://www.carbonbrief.org/analysis-how-low-sulphur-shipping-rules-are-affecting-global-warming/
21:46:46 From Clive Elsworth : Chlorophyl vs Cloud map: https://earthobservatory.nasa.gov/global-maps/MY1DMM_CHLORA/MODAL2_M_CLD_FR
21:50:08 From Dr Brian von Herzen : Reacted to |  (Click HERE to view the transcrript) (Click HERE to view the transcrript) |
|
|
|
|
|
| | # |
Name
(N/A = Not Available) |
Title |
Ref Date |
Date Added |
|---|
|
|
|
| Author | David Spratt and Ian Dunlop |
| Description | For climate change, 2023 was an “unprecedented” year, “absolutely gobsmackingly bananas” and “scary” and “frightening”. And that was what climate scientists said! The UN Secretary General called it the year in which humanity crossed into a new climate era — an age of “global boiling”.
Climate disruption shocked climate scientists in 2023. “Surprising. Astounding. Staggering. Unnerving. Bewildering. Flabbergasting. Disquieting. Gobsmacking. Shocking. Mind boggling,” said Prof. Ed Hawkins when September 2023 exceeded the previous September record by a huge 0.5°C.
The decline in Antarctic sea-ice extent was much greater than model projections, leading the National Snow and Ice Data Centre’s Walt Meier to exclaim: “It’s so far outside anything we’ve seen, it’s almost mind- blowing.”
Many records were set for new climate extremes — record heat, rainfall and floods — with some of it driven by the destabilisation of the polar jet stream. “We are hitting record breaking extremes much sooner than I expected. That’s frightening, scary, and concerning, and it really suggests that we’re not as aware of what’s coming as we thought we were,” said Sarah Perkins-Kirkpatrick of the University of NSW. |
| Contents | With devastating extreme heat and storms and floods, 2023 was the first year 1.5°C warmer than the 1850-1900 baseline, and both Antarctic sea-ice loss and record northern hemisphere sea-surface temperatures were way beyond the ranges projected by climate models.
Datasets of global temperatures vary a little depending on method, but two of the most significant are Berkeley Earth which put 2023 at 1.54°C above the pre-industrial (1850-1900) level, and Copernicus/ECMWF at 1.48°C.
Berkeley said that “a single year exceeding 1.5°C is a stark warning sign of how close the overall climate system has come to exceeding this Paris Agreement goal. With greenhouse gas emissions continuing to set record highs, it is likely that climate will regularly exceed 1.5°C in the next decade.”
2023 was notable for:
Global average warming hitting the 1.5°C mark, and new monthly records for global temperature every month from June to December. The October to December period was 1.74°C.
New national record high annual averages for an estimated 77 countries.
The first year that global average ocean surface temperatures exceeded 1°C, with once-in-a-century levels of warmth in the North Atlantic.
Two days in November when global average temperature, for the first time ever, reached 2°C above the pre-industrial levels.
Catastrophic flooding from Greece to Beijing to Vermont, and earlier in the year major flooding in New Zealand associated with a rain bomb and then cyclone Gabrielle.
Severe wildfires in Europe, Russia, Maui and North America; fires in Canada burned 18.5 million hectares of land.
The 2023 extremes were a shock. Prof. Katharine Hayhoe told the Guardian that: “We have strongly suspected for a while that our projections are underestimating extremes, a suspicion that recent extremes have proven likely to be true… We are truly in uncharted territory in terms of the history of human civilisation on this planet.”
Explanations for 2023 are incomplete, but warming is accelerating and 2024 is likely to be hotter
What happened in 2023 was not what scientists’ models anticipated at the beginning of the year and fell well outside the confidence intervals of any of the estimates. Carbon Brief says that “while there are a number of factors that researchers have proposed to explain 2023’s exceptional warmth, scientists still lack a clear explanation for why global temperatures were so unexpectedly high… researchers are just starting to disentangle the causes of the unexpected extreme global heat the world experienced in 2023”.
One person who has a clear view is the former NASA climate chief James Hansen who says that “the 1.5 degree limit is deader than a doornail” and warns that warming will accelerate to 1.7°C by 2030 and “2°C will be reached by the late 2030s”. |
|
|
|
|
| Description | Alarmingly, the rate of ocean warming has nearly doubled in the past decade compared to the 1990s, according to the study. |
| Contents | The world's oceans, often referred to as the "climate's heat sink," are now experiencing unprecedented rates of warming. Researchers at the University of New South Wales (UNSW Sydney) have uncovered the acceleration of ocean warming and have pinpointed the areas that are absorbing the most heat. |
|
|
|
|
| Author | John Nissen |
| Description | Framing
IPCC and the fossil fuel industry
Climate crisis looms
Denial of need for cooling intervention
Cover-up of global warming crisis
Cover-up of Arctic situation
Best interest for humanity
Hope for the future
The urgency for cooling intervention could not be greater, nor the rewards.
|
|
|
|
|
Agenda
John N – BBC News says 1.5C will arrive twice as soon as previously thought.
- Letter to Guardian re Hansen Global warming in pipeline paper
Chris V – Report from Chatham House – focusing on 1.5 is flawed, focus on tipping points
Hugh H – MCB and controlling hurricanes
Chris V – Compendium of interventions – in the chat
Sev – NOAC website progress – integration of Blue Cooling Initiative, HPAC ,PRAG
Ron – Comments on bunker fuel letter?
Clive – Just Have A Think – recent video very good.
– Radiative forcing to -0.26 W/m2 waterfall chart demo.
Chat
20:06:23 From Chris Vivian - GESAMP WG 41 : Carbon emissions threaten 1.5C climate threshold sooner than thought – report - https://www.bbc.co.uk/news/science-environment-67242386
20:07:16 From Chris Vivian - GESAMP WG 41 : Global warming: Why focusing on 1.5C is flawed - https://www.chathamhouse.org/publications/the-world-today/2023-10/global-warming-why-focusing-15c-flawed?utm_source=Chatham%20House&utm_medium=email&utm_campaign=14169358_CH%20-%20Content%20Newsletter%20-%2017.10.23&utm_content=Global-Title&dm_i=1S3M,8FP5A,NODY6,YTVON,1
20:10:08 From Chris Vivian - GESAMP WG 41 : FROZEN ARCTIC: Compendium of interventions to slow down, halt, and reverse the effects of climate change in the Arctic and northern regions - https://new.uarctic.org/media/to0bjpal/frozen-arctic-rra-compendium.pdf
20:10:48 From Ron Baiman : First part of Sharpe's book is very good on risk analysis and climate.
20:12:43 From Ron Baiman : Bunker fuel letter link: https://docs.google.com/document/d/1WNsRI8GbyZgdso39ptKuKFI2HA6ZuBni/edit?usp=sharing&ouid=116465941111195452408&rtpof=true'sd=true
20:13:23 From Herb Simmens : The Nov 16th HPAC meeting will feature Mike McCracken discussing the differing approaches to addressing risk that various professions and institutions take
20:15:28 From Herb Simmens : This Thursday Nov 2 HPAC meeting at 3:30 PM EDT 19:30 PM GMT will feature Doug MacMartin of Cornell on SRM/SAI..
20:17:00 From Robert Chris : https://www.nature.com/articles/s41558-023-01848-5
20:19:42 From Herb Simmens : This blog post has a link to the paper in the first sentence https://scienceisshiny.wordpress.com/2023/10/30/carbon-budgets-how-hard-is-the-paris-agreement-now/
20:21:37 From Bill Chapman, Brooklyn : I was horrified when Trump and some Republicans started talking about |  (Click HERE to view the transcrript) (Click HERE to view the transcrript) |
|
|
|
|
| Description | West Antarctica is headed for decades of rapid melting no matter how quickly humans cut greenhouse gas emissions, and 2023 shattered records for missing sea ice around the continent. |
| Contents | A trio of new scientific analyses about the loss of ice in Antarctica paint a picture of a continent in trouble. Sea ice is disappearing, gigantic portions of the West Antarctic ice sheet are crumbling and even relatively stable East Antarctica is showing worrying changes. |
|
|
|
|
|
| | # |
Name
(N/A = Not Available) |
Title |
Ref Date |
Date Added |
|---|
|
|
|
Agenda
Chris GESAMP Report Press Release from IMO
BBC Report Big Seaweed Farm Sargassum South Atlantic
Sev - Trichodesmium cyanobacterium
Cost comparison brightening planet v cutting emissions
COP27 "“ Ocean Pavilion
Concert
From Chris Vivian to Everyone: 07:09 AM
IMP Press Release - https://www.imo.org/en/MediaCentre/PressBriefings/pages/Marine-geoengineering.aspx
From mycomputer to Everyone: 07:09 AM
https://en.wikipedia.org/wiki/Trichodesmium
From Chris Vivian to Everyone: 07:09 AM
BBC News story about Seafields https://www.bbc.co.uk/news/science-environment-63200589
From Me to Everyone: 07:30 AM
https://oceanvisions.org/our-programs/macroalgaeresearchframework/
From Chris Vivian to Everyone: 07:31 AM
https://doi.org/10.1038/nature09950
From Chris Vivian to Everyone: 07:53 AM
Ocean deserts are growing - https://doi.org/10.1038/news.2008.795
Regional Geoengineering Using Tiny Glass Bubbles Would Accelerate the Loss of Arctic Sea Ice - https://doi.org/10.1029/2022EF002815
From Me to Everyone: 08:00 AM
https://oilprice.com/Energy/Energy-General/Chevron-CEO-Blames-Climate-Policies-For-Global-Energy-Crisis.html
From Chris Vivian to Everyone: 08:03 AM
You might be interested in this post - Climate scientists: concept of net zero is a dangerous trap - https://theconversation.com/climate-scientists-concept-of-net-zero-is-a-dangerous-trap-157368
http://newenergytimes.com/v2/sr/companies/RussGeorge/2013/20140224HSRC-vs-Russ-George-counterclaim.pdf
http://newenergytimes.com/v2/sr/companies/RussGeorge/Russ-George-Low-Energy-Nuclear-Reaction-Research-LENR-and-Plankton-Carbon-Credit.shtml |  (Click HERE to view the transcrript) (Click HERE to view the transcrript) |
|
|
|
|
Special guests, Stephen Salter and Peter Wadhams, join Paul Beckwith and Regina Valdez to discuss Marine Cloud Brightening. Stephen Salter is one of the leading voices of the Marine Cloud Brightening (MCB) movement.
As average global temperatures rise, increasing the reflectivity of clouds over the ocean has been studied as a geoengineering method to reflect more solar radiation away from the Earth thus reducing and reversing the warming caused by excess CO2 concentration in the atmosphere.
This CEF program was recorded at COP26, Glasgow, Scotland, in the Durdle Door press conference room on November 4th, 2021, and published on December 13th, 2021. This presentation is brought to you through a collaboration with FacingFuture.TV https://www.youtube.com/c/FacingFuture.
Topics discussed include the following:
- Why some clouds, such as cumulonimbus are dark, and some are light
- Using nozzles installed on ships to spray very small drops of filtered seawater 0.8 microns in diameter.
- Ships spraying filtered droplets of sea water could be deployed to reverse sea level rise and/or save the Arctic sea ice.
- Governments of countries could decide on targets for sea-surface temperatures
- Cost estimates to deploy a fleet of 800 spray vessels/ships
- Advantages of MCB as a geoengineering technique to prevent the worst extremes of climate change
- How we can change the reflectivity of clouds in areas that will most benefit the Earth.
Links:
Earth is dimming due to climate change
https://news.agu.org/press-release/earth-is-dimming-due-to-climate-change/
Special Guests:
Stephen Salter - Emeritus Professor of Engineering Design at the University of Edinburgh and is responsible for creating the concept of the mechanical enhancement of clouds to increase their reflectivity. Professor Salter is a Member of the Order of the British Empire.
Dr. Peter Wadhams - ScD, is emeritus professor of Ocean Physics, and Head of the Polar Ocean Physics Group in the Department of Applied Mathematics and Theoretical Physics, University of Cambridge. He is best known for his work on sea ice.
Regular Panelists:
Paul Beckwith
- Climate Systems Scientist. Professor at the University of Ottawa in the Paleoclimatology Laboratory as well as at Carleton University
Regina Valdez
- Program Director, Climate Reality Project, NYC. GreenFaith Fellow and LEED Green Associate
Video Production:
UNFCCC
COP26 Media Crew in the Durdle Door Press Conference Room
Charles Gregoire
- Electrical Engineer, Webmaster and IT prime for FacingFuture.Earth \u0026 the Climate Emergency Forum; Climate Reality Leader
Heidi Brault
- Video production and website assistant, Organizer and convener, Metadata technician, BA (Psychology), COP26 team lead for FacingFuture.Earth and the Climate Emergency Forum; Climate Reality Leader
Thanks to the following organizations for making this program possible:
- Buddhist Tzu-Chi Foundation
- Interfaith Center for Sustainable Development
- International Society of Ecological Economics
Attributions
Background Music:
- Title: Through the City II
- Author: Crowander
- Source: Free Music Archive
- License: CC BY-NC 4.0
Image and Video: https://climateemergencyforum.org/assets/attributions/2021-12-13-marine-cloud-brightening.html |  (Click HERE to view the transcrript) (Click HERE to view the transcrript) |
|
|
|
| 3 |
c8Ko60kXk6w (16) |
On the Deployment of Ocean Spraying Vessels to Brighten Marine Clouds to Cool the Planet: 4 of 4 |
6/23/21 |
11/20/23 |
|
|
|
| 3 |
c8Ko60kXk6w (16) |
On the Deployment of Ocean Spraying Vessels to Brighten Marine Clouds to Cool the Planet: 4 of 4 |
6/23/21 |
11/20/23 |
|
I was recently in a great video discussion with Peter Wadhams and Stephen Salter, hosted by Metta Spencer, to hash out the cloud brightening technique as conceptualized by Emeritus Professor Stephen Salter in the Engineering and Design Department at the University of Edinburgh over the last couple of decades.
Marine Cloud Brightening (MCB) has the potential to cool the planet in a highly controllable fashion. Essentially, sea water is pumped to high pressure through nozzles where it generates water jets that then break apart (via Rayleigh instability) to form tiny water droplets. The nozzle size, number of nozzles, water pressure, etc"¦ are engineered to produce water droplets of 800 nm size (0.8 micron) so that when the water evaporates we are left with 200 nm salt crystals. These salt crystals are then transported within the turbulent boundary layer above the surface of the ocean up to heights about 1 km to 1.5 km where they act as cloud condensation nuclei, ensuring that the clouds that do form are of extremely high albedo (reflectivity) and thus can reflect enough incoming sunlight to cool the surface of the Earth.
The spray nozzles are transported around the oceans of the planet by hydrofoil ships powered by the wind using so-called Flettner Rotors. The ships are sailed to specific areas of the ocean at specific times of the year to brighten the clouds in specific regions to get the desired regional cooling, for example to reduce Atlantic Basin hurricane strength, protect coral reefs, cool the Arctic enough to restore Arctic Sea Ice, and!or modify monsoons or redistribute rainfall to reduce droughts or torrential rainfalls.
This technology has enormous potential to cool the planet enough to buy us time to slash fossil fuel emissions and deploy carbon removal technologies.
Please donate to http://paulbeckwith.net to support my research and videos on abrupt climate change science, consequences, and solutions. |  (Click HERE to view the transcrript) (Click HERE to view the transcrript) |
|
|
|
|
|
| | # |
Name
(N/A = Not Available) |
Title |
Ref Date |
Date Added |
|---|
|
|
|
| Description | This is the second article in a two-part series. Read the first part here.
Climate model scenarios similar to current policies project 2°C of warming before 2050; if James Hansen is right (see Part 1) and warming sharply accelerates, it could be a decade sooner. These outcomes will be driven by the high energy imbalance, continuing high emissions, the accelerating accumulation of heat in the oceans, and decreases in short-term aerosol cooling. |
| Contents | Several years ago a group of eminent scientists proposed a “carbon law”, which said that keeping warming to 2°C required emissions to be halved every decade from 2020 onwards, including a halving between 2020 and 2030, plus some carbon drawdown. Instead, the level of greenhouse gases and coal use both hit record highs in 2023. And the largest national fossil fuel producers plan to keep on expanding production As a result, current government plans worldwide will likely result in emissions in 2050 almost as high as they are today, according to the UN Environment Programme’s 2023 Production Gap report.
Other analyses are broadly consistent:
The International Energy Agency finds that stated national policies will result in oil and gas production in 2050 as high as 2020; with coal halved.
The OECD finds that a world economy four times larger than today is projected to need 80% more energy in 2050; and without new policy action and the global energy mix in 2050 will not differ significantly from today.
The intentions of the world’s five largest fossil fuel producers are clear — and civilisation-threatening — as reported by the UN:
In China, oil production is projected to be flat to 2050, but gas will increase more than 60 percent from 2020 to 2050, while coal use will remain high till 2030 then decline sharply.
In the United States, oil production will grow and then remain at record levels to 2050, and gas is projected to continuously and significantly increase to 2050; whilst coal will drop by half.
Projections for Russia are available only to 2035, with coal and gas production projected to increase significantly, while oil remains flat.
In Saudi Arabia, oil production is projected to grow by 26 to 47 percent by 2050, with gas up 40 percent between 2019 and 2050. Together they make up half of the Saudi economy.
And in Australia, one of the world’s top two liquified natural gas and coal exporters, gas production is projected to stay above the current level for the next 15 years, with coal remaining high over the same period, above 450 million metric tons annually.
We are heading towards 3–4°C.
This outlook suggests Earth is heading towards 3°C of warming and perhaps a good deal more, because current climate models which project warming of around 2.7°C do not adequately account for all the system-level reinforcing feedbacks.
In 2021, the pre-eminent UK international affairs think-tank Chatham House said a “plausible worst-case scenario” is 3.5°C or more, which could be an underestimate if tipping points are reached sooner than the orthodox science suggests. This now seems to be the reality.
A clear majority of scientists expected warming of more than 3°C, and 82% expected to see catastrophic impacts of climate change in their lifetime, according to a 2021 survey by the journal Nature.
Questions about the size of the aerosol forcing, and the related issue of how sensitive the climate is to changes in greenhouse gases, remain an issue of scientific contention.
New climate history research published in December 2023, based on a study of the last 66 million years, concluded that global temperature may be more sensitive to CO2 levels than current models estimate. It showed that the last time CO2 levels were as high as today was around 14 million year ago, which is longer than previous estimates, and that climate sensitivity — the amount of warming resulting from a doubling of atmospheric CO2 — may be between between 5°C and 8°C, compared to the IPCC orthodoxy of 1.5–4.5°C.
The level of greenhouse gases is currently around 560 parts per million, double the pre-industrial level. Some of those gases such as methane are short-lived so this level of forcing is not written in stone, but nevertheless if Hansen et al. are right that a doubling may lead to around 4–5°C of warming, then another 30 years of high emissions means humans will have created an increasingly unliveable planet.
Has the impact of aerosols been widely understood? In what the New York Times described as “an eye-opening Nature commentary”, Geeta Persad and her colleagues wrote in late 2022 that “overall, vast emissions of aerosols since the start of the industrial age have had a profound cooling effect” and that without them “the global warming we see today would be 30 to 50 percent greater”, warning that “the impacts of aerosols on climate risk are often ignored”.
In 2018, a group of eminent scientists explored the potential — once warming had exceeded the 1.5–2°C range — for self-reinforcing positive feedbacks in major elements of the climate system to push passed a planetary threshold that would prevent temperature stabilisation, and drive the system to a “Hothouse Earth”. They warned that “we are in a climate emergency… this is an existential threat to civilisation”.
The 2023 State of the Climate Report: Entering uncharted territory warned of: “potential collapse of natural and socioeconomic systems in such a world [of 2.6°C warming] where we will face unbearable heat, frequent extreme weather events, food and fresh water shortages, rising seas, more emerging diseases, and increased social unrest and geopolitical conflict.”
Whatever the words, the understanding is widely shared that contemporary nations and societies, and likely the global social system, are heading towards collapse. “If we carry on the way we are going now, I can’t see this civilisation lasting to the end of this century”, says Professor Tim Lenton. The US Defence Secretary Lloyd Austin III calls the risks “existential”.
Opening the Innovation Zero Congress in London in May 2023, Potsdam Institute Director Prof. Johan Rockstrom described the path we are on:
“2.5°C global mean surface temperature rise is a disaster. It’s something that humanity has absolutely no evidence that we can cope with… [There] would be a 10-metre sea-level rise. There would be a collapse of all the big biomes on planet Earth – the rainforest, many of the temperate forests – abrupt thawing of permafrost, we will have complete collapse of marine biology… Over one-third of the planet around the equatorial regions will be uninhabitable because you will pass the threshold of health, which is around 30°C. It’s only in some parts of the Sahara Desert today that has that kind of average temperature.”
Chatham House’s Climate Risk Assessment 2021 concludes that by 2050 global food demand would be 50% higher, but crop yields may drop by 30%. As desertification spreads across the dry sub-tropics, and one-third of the planet experiences unprecedented heat, it is not difficult to see why they concluded that cascading climate impacts will “drive political instability and greater national insecurity, and fuel regional and international conflict”.
What is worse is the setback to climate action posed by current conflicts and military posturing in Europe, the Middle East and east Asia, which are huge political distractions from dealing with the greatest threat to humanity, and all of which have the potential to spread more widely.
To maintain military flexibility, the US insisted in 1997 that direct military carbon emissions be excluded from international carbon accounting. Those emissions, around 5 percent of the total global, are far less than the indirect emissions from conflict, as recent estimates here and here indicate.
Projections show that by 2100 the expansion of the Sahara due to desertification will embrace Israel/Palestine, as well as spreading across the Mediterranean into Spain, Italy, Greece and Turkey (see map).
The Australian Prime Minister has finally spoken out about the escalating climate threat whilst inspecting damage from the recent Queensland floods: “All of this is a reminder that the science told us that climate change would mean there would be more extreme weather events and they would be more intense. And unfortunately, we are seeing that play out with the number of events that we’re having to deal with right around Australia”.
Just so, except that in common with leaders globally, the Australian government continues to have its head stuck in the sand about the real risks climate change now represents. It refuses to release an intelligence assessment of climate-security risks, and has fumbled a domestic climate risk assessment.
As a result, the community remains ill-informed and unprepared for what is coming. |
|
|
|
|
| Description | Global heating of the Earth system is unequivocal. However, detecting an acceleration of Earth heating has remained elusive to date, despite suggestive evidence of a potential increase in heating rates. In this study, we demonstrate that since 1960, the warming of the world ocean has accelerated at a relatively consistent pace of 0.15 ± 0.05 (W/m2)/decade, while the land, cryosphere, and atmosphere have exhibited an accelerated pace of 0.013 ± 0.003 (W/m2)/decade. This has led to a substantial increase in ocean warming, with a magnitude of 0.91 ± 0.80 W/m2 between the decades 1960-1970 and 2010-2020, which overlies substantial decadal-scale variability in ocean warming of up to 0.6 W/m2. Our findings withstand a wide range of sensitivity analyses and are consistent across different observation-based datasets. The long-term acceleration of Earth warming aligns qualitatively with the rise in CO2 concentrations and the decline in aerosol concentration during the same period, but further investigations are necessary to properly attribute these changes. |
| Contents | In the past 150 years, Earth's climate has been warming at a rate that is unprecedented in at least the last 2000 years1. This human-caused warming has caused widespread adverse impacts and related losses and damages to nature and people, which will continue in the future as global climate continues to warm2. Detecting changes in the rate of warming is crucial for informed decision-making in international climate negotiations, with the aim of limiting global warming to specific levels. However, it remains a significant challenge to detect such changes due to the substantial internal variability of the climate system on a decadal scale (e.g., ref. 3). In this paper, we address this challenge by examining the global heat accumulation rate across the entire climate system, including the ocean, atmosphere, cryosphere, and land. By focusing on this integrated view, rather than solely relying on changes in global mean surface temperature, we can mitigate the impact of variability and gain a more comprehensive understanding4,5. Global heat accumulation in the climate system, resulting from the current positive Earth's Energy Imbalance (EEI) at the top of the atmosphere, is primarily dominated by changes in Global Ocean Heat Content (GOHC)4. GOHC changes account for approximately 90% of the total heat increase in the past fifty years, while land heating, ice melting, and atmospheric warming contribute around 5%, 3%, and 1% respectively6,7,8. Several studies have indicated an increase in the global heat accumulation rate in recent decades, with values rising from 0.50 [0.32 to 0.69] W/m2 during the period 1971-2006 to 0.79 [0.52 to 1.06] W/m2 (90% confidence interval) for the period 2006-2018 (ref.4,6,7,8,9,10,11,12,13,14,15,16,17,18,19,20,21,22 and Fig. 1). Some studies have even suggested a potential doubling of EEI in the last decade compared to the previous one6,17. Figure 1 |
|
|
|
|
| Description | James E. Hansen ABSTRACT
Improved knowledge of glacial-to-interglacial global temperature change implies that fastfeedback equilibrium climate sensitivity (ECS) is 1.2 ± 0.3°C (2s) per W/m2
. Consistent analysis
of temperature over the full Cenozoic era – including “slow” feedbacks by ice sheets and trace
gases – supports this ECS and implies that CO2 was about 300 ppm in the Pliocene and 400 ppm
at transition to a nearly ice-free planet, thus exposing unrealistic lethargy of ice sheet models.
Equilibrium global warming including slow feedbacks for today’s human-made greenhouse gas
(GHG) climate forcing (4.1 W/m2
) is 10°C, reduced to 8°C by today’s aerosols. Decline of
aerosol emissions since 2010 should increase the 1970-2010 global warming rate of 0.18°C per
decade to a post-2010 rate of at least 0.27°C per decade. Under the current geopolitical approach
to GHG emissions, global warming will likely pierce the 1.5°C ceiling in the 2020s and 2°C
before 2050. Impacts on people and nature will accelerate as global warming pumps up
hydrologic extremes. The enormity of consequences demands a return to Holocene-level global
temperature. Required actions include: 1) a global increasing price on GHG emissions, 2) EastWest cooperation in a way that accommodates developing world needs, and 3) intervention with
Earth’s radiation imbalance to phase down today’s massive human-made “geo-transformation”
of Earth’s climate. These changes will not happen with the current geopolitical approach, but
current political crises present an opportunity for reset, especially if young people can grasp their
situation. |
|
|
|
|
| Description | We need a new way of talking about global warming. UN Secretary General António
Guterres underscored this when he said the “era of global boiling” has arrived. Although
we have made remarkable progress on a very complex problem over the past thirty years,
we have a long way to go before we can keep the global temperature increase to below 2°C
relative to the pre-industrial times. Climate models suggest that this next decade is critical
if we are to avert the worst consequences of climate change. The world must continue
to reduce greenhouse gas emissions, and find ways to adapt and build resilience among
vulnerable communities. At the same time, we need to find new ways to remove carbon
dioxide from the atmosphere in order to chart a “net negative” emissions pathway. Given
their large capacity for carbon storage, the oceans must be included in consideration of
our multiple carbon dioxide removal (CDR) options (1).
This report focused on ocean iron fertilization (OIF) for marine CDR. This is by no
means a new scientific endeavor. Several members of ExOIS (Exploring Ocean Iron
Solutions) have been studying this issue for decades, but the emergence of runaway climate
impacts has motivated this group to consider a responsible path forward for marine
CDR. That path needs to ensure that future choices are based upon the best science and
social considerations required to reduce human suffering and counter economic and ecological
losses, while limiting and even reversing the negative impacts that climate change
is already having on the ocean and the rest of the planet.
Prior studies have confirmed that the addition of small amounts of iron in some parts
of the ocean is effective at stimulating phytoplankton growth. Through enhanced photosynthesis,
carbon dioxide can not only be removed from the atmosphere but a fraction
can also be transferred to durable storage in the deep sea. However, prior studies were
not designed to quantify how effective this storage can be, or how wise OIF might be as
a marine CDR approach. |
| Contents | needed to answer critical questions about the potential efficiency and ecological impacts
of marine CDR (http://oceaniron.org). Owing to concerns surrounding the ethics of marine
CDR, ExOIS is organized around a responsible code of conduct that prioritizes activities
for the collective benefit of our planet with an emphasis on open and transparent
studies that include public engagement (2; see inset pg. 3).
Our goal is to establish open-source conventions for implementing OIF for marine
CDR that can be assessed with appropriate monitoring, reporting, and verification
(MRV) protocols, going beyond just carbon accounting, to assess ecological and other
non-carbon environmental effects (eMRV). As urgent as this is, it will still take 5 to 10
years of intensive work and considerable resources to accomplish this goal.
We present here a “Paths Forward’’ report that stems from a week-long workshop held
at the Moss Landing Marine Laboratories in May 2023 that was attended by international
experts spanning atmospheric, oceanographic, and social sciences as well as legal specialists
(see inside back cover). At the workshop, we reviewed prior OIF studies, distilled
the lessons learned, and proposed several paths forward over the next decade to lay the
foundation for evaluating OIF for marine CDR. Our discussion very quickly resulted in
a recommendation for the need to establish multiple “Ocean Iron Observatories’’ where,
through observations and modeling, we would be able to assess with a high degree of
certainty both the durable removal of atmospheric carbon dioxide—which we term the
“centennial tonne”—and the ecological response of the ocean.
3 PATHS FORWARD FOR EXPLORING OCEAN IRON FERTILIZATION
In a five-year phase I period, we prioritize five major research activities:
1. Next generation field studies
Studies of long-term (durable) carbon storage will need to be longer (year or more) and
larger (>10,000 km2) than past experiments, organized around existing tools and models, but
with greater reliance on autonomous platforms. While prior studies suggested that ocean
systems return to ambient conditions once iron infusion is stopped, this needs to be verified.
We suggest that these next field experiments take place in the NE Pacific to assess the
processes controlling carbon removal efficiencies, as well as the intended and unintended
ecological and geochemical consequences.
2. Regional, global and field study modeling
Incorporation of new observations and model intercomparisons are essential to accurately
represent how iron cycling processes regulate OIF effects on marine ecosystems and carbon
sequestration, to support experimental planning for large-scale MRV, and to guide decision
making on marine CDR choices.
3. New forms of iron and delivery mechanisms
Rigorous testing and comparison of new forms of iron and their potential delivery
mechanisms is needed to optimize phytoplankton growth while minimizing the financial
and carbon costs of OIF. Efficiency gains are expected to generate responses closer to those
of natural OIF events.
4. Monitoring, reporting, and verification
Advances in observational technologies and platforms are needed to support the development,
validation, and maintenance of models required for MRV of large-scale OIF deployment. In
addition to tracking carbon storage and efficiency, prioritizing eMRV will be key to developing
regulated carbon markets.
5. Governance and stakeholder engagement
Attention to social dimensions, governance, and stakeholder perceptions will be essential
from the start, with particular emphasis on expanding the diversity of groups engaged in
marine CDR across the globe. This feedback will be a critical component underlying future
decisions about whether to proceed, or not, with OIF for marine CDR.
Paramount in the plan is the need to move carefully. Our goal is to conduct these five activities in parallel
to inform decisions steering the establishment of ocean iron observatories at multiple locations in phase
II. When completed, this decadal plan will provide a rich knowledge base to guide decisions about if,
when, where, and under what conditions OIF might be responsibly implemented for marine CDR.
The consensus of our workshop and this report is that now is the time for actionable studies to begin.
Quite simply, we suggest that some form of marine CDR will be essential to slow down and reverse the
most severe consequences of our disrupted climate. OIF has the potential to be one of these climate
mitigation strategies. We have the opportunity and obligation to invest in the knowledge necessary to
ensure that we can make scientifically and ethically sound decisions for the future of our planet. |
|
|
|
| 5 |
egusphere.copernicus.org/... |
EGUsphere - The interaction of Solar Radiation Modification and Earth System Tipping Elements |
10/10/23 |
11/5/23 |
|
|
|
| 5 |
egusphere.copernicus.org/... |
EGUsphere - The interaction of Solar Radiation Modification and Earth System Tipping Elements |
10/10/23 |
11/5/23 |
|
| Description | Abstract. The avoidance of hitting tipping points is often considered a key benefit of Solar Radiation Modification (SRM) techniques, however, the physical science underpinning this has thus far not been comprehensively assessed. This review assesses the available evidence for the interaction of SRM with a number of earth system tipping elements in the cryosphere, the oceans, the atmosphere and the biosphere , with a particular focus on the impact of SAI. We review the scant available literature directly addressing the interaction of SRM with the tipping elements or for closely related proxies to these elements. However, given how limited this evidence is, we also identify and describe the drivers of the tipping elements, and then assess the available evidence for the impact of SRM on these. We then briefly assess whether SRM could halt or reverse tipping once feedbacks have been initiated. Finally, we suggest pathways for further research. We find that SRM mostly reduces the risk of hitting tipping points relative to same emission pathway scenarios without SRM, although this conclusion is not clear for every tipping element, and large uncertainties remain. |
| Contents | <p><strong class= |
|
|
|
|
|
| | # |
Name
(N/A = Not Available) |
Title |
Ref Date |
Date Added |
|---|
|
|
|
Agenda
Doug "“ Does climate sensitivity matter in the short term?
Sev "“ Greenknights "“ putting Buoyant flakes before US Congress
Rebecca "“ MCB Great barrier reef "“ status Stephen?
Doug "“ Beneath the Polar Sun
Doug "“ Kamchatka volcano "“ effects measurement?
Clive "“ Greenland "“ grayland BBC video.
Peter - pictures of Greenland
Mannajo "“ comment on Greenland
Sev "“ Cartoon
Chat
21:01:22 From Sev Clarke : https://www.theguardian.com/commentisfree/2023/apr/17/hey-dont-krill-yourself-humanity?utm_term=643d18a6ed47af625f01d0443e5baa44\u0026utm_campaign=BestOfGuardianOpinionAUS\u0026utm_source=esp\u0026utm_medium=Email\u0026CMP=opinionau_email
21:01:27 From Sev Clarke : https://docs.google.com/document/d/1dpytym6Pq3g1UkrnSLE7zHRxYyimlxfkj7jYGzaX95I/edit
21:15:01 From Doug Grandt (Vermont) : PBS's movie |  (Click HERE to view the transcrript) (Click HERE to view the transcrript) |
|
|
|
|
| Description | Tipping elements are components of the Earth system which may respond nonlinearly to anthropogenic climate change by transitioning toward substantially different long-term states upon passing key thresholds or “tipping points.” In some cases, such changes could produce additional greenhouse gas emissions or radiative forcing that could compound global warming. Improved understanding of tipping elements is important for predicting future climate risks and their impacts. Here we review mechanisms, predictions, impacts, and knowledge gaps associated with 10 notable Earth system components proposed to be tipping elements. We evaluate which tipping elements are approaching critical thresholds and whether shifts may manifest rapidly or over longer timescales. Some tipping elements have a higher risk of crossing tipping points under middle-of-the-road emissions pathways and will possibly affect major ecosystems, climate patterns, and/or carbon cycling within the 21st century. However, literature assessing different emissions scenarios indicates a strong potential to reduce impacts associated with many tipping elements through climate change mitigation. The studies synthesized in our review suggest most tipping elements do not possess the potential for abrupt future change within years, and some proposed tipping elements may not exhibit tipping behavior, rather responding more predictably and directly to the magnitude of forcing. Nevertheless, uncertainties remain associated with many tipping elements, highlighting an acute need for further research and modeling to better constrain risks. |
| Contents | Plain Language Summary
In recent years, discussions of climate change have shown growing interest in “tipping elements” of the Earth system, also imprecisely referred to as “tipping points.” This refers to Earth system components like the tropical rainforests of Amazonia or the Greenland and Antarctic ice sheets which may exhibit large-scale, long-term changes upon reaching critical global warming, greenhouse gas, or other thresholds. Once such thresholds are passed, some tipping elements could in turn produce additional greenhouse gas emissions or change the Earth's energy balance in ways that moderately reinforce warming. In this review, we summarize the current state of scientific knowledge on 10 systems that some have referred to as potential tipping elements of the climate system. We describe the mechanisms important to each system, highlight the response of these systems to climate change so far, and explain the dynamics of potential future changes that these systems could undergo in response to further climate change. Overall, even considering remaining scientific uncertainties, tipping elements will influence future climate change and may involve major impacts on ecosystems, climate patterns, and the carbon cycle starting later this century. Aggressive efforts to stabilize climate change could significantly reduce such impacts. |
|
|
|
|
Agenda
Chris "“ Mangroves to save the climate - oversold
Chris - Draft AGU position status
Sev - Climate interventions without proper approval
Doug - Feedback on Elephant in room visual
Peter W "“ Burnt forest in Sardinia vs Darkened Greenland ice
Brian - The Earthshot prize winners
Chris - Steve Keen"™s webinar on neoclassical economists getting climate economics wrong
Chat
20:24:06 From Chris Vivian : Seychelles https://www.bbc.com/news/world-africa-63901644. Also, see these articles about blue carbon https://www.frontiersin.org/articles/10.3389/fclim.2022.853666 and https://www.aweimagazine.com/article/challenges-for-blue-carbon-solutions/
20:30:18 From DV Henkel-Wallace : I can't find the Shaun Fitzgerald zoom link -- could someone put in chat please?
20:31:16 From Chris Vivian : AGU https://www.agu.org/Share-and-Advocate/Share/Policymakers/Position-Statements/Draft-Climate-Intervention
20:31:50 From Sev Clarke : Shaun Fitzgerald https://us02web.zoom.us/j/82355048687 |  (Click HERE to view the transcrript) (Click HERE to view the transcrript) |
|
|
|
|
Agenda
Aaron "“ Greenland blowing up? Arctic conditions
JM "“ Predicted loss of clouds?
Sev "“ Separating humidity from nuclei
CE/Franz Nozzle design "“ Shaun?
Grant "“ Crack in conversation on emissions/CDR/SRM
RT "“ Pakistand Floods "“ S.Salter |  (Click HERE to view the transcrript) (Click HERE to view the transcrript) |
|
|
|
| 5 |
acp.copernicus.org/articl... |
Ice melt, sea level rise and superstorms: evidence from paleoclimate data, climate modeling, and modern observations that 2 ?C global warming could be dangerous |
3/22/26 |
1/6/24 |
|
|
|
| 5 |
acp.copernicus.org/articl... |
Ice melt, sea level rise and superstorms: evidence from paleoclimate data, climate modeling, and modern observations that 2 ?C global warming could be dangerous |
3/22/26 |
1/6/24 |
|
| Author | James Hansen1 , Makiko Sato1 , Paul Hearty2 , Reto Ruedy3,4 , Maxwell Kelley3,4 , Valerie Masson-Delmotte5 , Gary Russell4 , George Tselioudis4 , Junji Cao6 , Eric Rignot7,8 , Isabella Velicogna7,8 , Blair Tormey9 , Bailey Donovan10 , Evgeniya Kandiano11, Karina von Schuckmann12, Pushker Kharecha1,4 , Allegra N. Legrande4 , Michael Bauer4,13 , and Kwok-Wai Lo3,4 |
| Description | Abstract. We use numerical climate simulations, paleoclimate data, and modern observations to study the effect of
growing ice melt from Antarctica and Greenland. Meltwater tends to stabilize the ocean column, inducing amplifying
feedbacks that increase subsurface ocean warming and ice
shelf melting. Cold meltwater and induced dynamical effects
cause ocean surface cooling in the Southern Ocean and North
Atlantic, thus increasing Earth’s energy imbalance and heat
flux into most of the global ocean’s surface. Southern Ocean
surface cooling, while lower latitudes are warming, increases
precipitation on the Southern Ocean, increasing ocean stratification, slowing deepwater formation, and increasing ice
sheet mass loss. These feedbacks make ice sheets in contact
with the ocean vulnerable to accelerating disintegration. We
hypothesize that ice mass loss from the most vulnerable ice,
sufficient to raise sea level several meters, is better approximated as exponential than by a more linear response. Doubling times of 10, 20 or 40 years yield multi-meter sea level
rise in about 50, 100 or 200 years. Recent ice melt doubling
times are near the lower end of the 10–40-year range, but
the record is too short to confirm the nature of the response.
The feedbacks, including subsurface ocean warming, help
explain paleoclimate data and point to a dominant Southern
Ocean role in controlling atmospheric CO2, which in turn exercised tight control on global temperature and sea level. The
millennial (500–2000-year) timescale of deep-ocean ventilation affects the timescale for natural CO2 change and thus
the timescale for paleo-global climate, ice sheet, and sea |
|
|
|
|
|
| | # |
Name
(N/A = Not Available) |
Title |
Ref Date |
Date Added |
|---|
|
|
|
| Author | William J. Ripple,1,2,7 Christopher Wolf,1,7, * Timothy M. Lenton,3 Jillian W. Gregg,4 Susan M. Natali,5 Philip B. Duffy,5 Johan Rockstro¨ m,6 and Hans Joachim Schellnhuber6 |
| Description | Many feedback loops significantly increase warming due to greenhouse gas emissions. However, not all of these feedbacks are fully accounted for in climate models. Thus, associated mitigation pathways could fail to sufficiently limit temperatures. A targeted expansion of research and an accelerated reduction of emissions
are needed to minimize risks. |
| Contents | A new report written by an international team of researchers, including scientists from Oregon State University (OSU), warns of many risky climate feedback loops and the need for action in both research and policy. Published in the journal One Earth today (February 17), the report states that partly due to amplifying climate feedbacks, "a very rapid drawdown in emissions will be required to limit future warming."
Researchers from the United States and Europe listed and described 41 climate feedback loops that have major implications for the outlook on climate change. Climate feedback loops are processes that can either amplify or diminish the effects of our greenhouse gas emissions, initiating a cyclical chain reaction that keeps repeating again and again. There are many large amplifying feedbacks that accentuate warming. In total, the researchers identified 27 amplifying feedbacks, 7 dampening feedbacks, and 7 uncertain feedbacks. |
|
|
|
|
|
| | # |
Name
(N/A = Not Available) |
Title |
Ref Date |
Date Added |
|---|
|
|
| 1 |
Word |
Climate crisis “out of control” (Letter sent to the Guardian containing SAI proposal but not published) |
10/10/23 |
11/26/23 |
|
|
|
| 1 |
Word |
Climate crisis “out of control” (Letter sent to the Guardian containing SAI proposal but not published) |
10/10/23 |
11/26/23 |
|
| Author | John Nissen (On behalf of the Planetary Restoration Action Group (PRAG)) |
| Description | We have a historically unprecedented climate emergency. The planet is now heating so fast that the Paris-agreed ceiling of 1.5°C global mean temperature is liable to be reached next year and 2°C within a decade or two. This rapid heating will be boosted by Arctic meltdown: with less sunshine reflected by snow and ice, and with the release of the potent greenhouse gas, methane, from thawing permafrost. Arctic meltdown also affects the jet stream: we can expect the double-whammy of increased global heating and stuck jet stream patterns to produce ever more extreme heatwaves, droughts and wildfires. The climate crisis is indeed spiralling “out of control” (the Guardian headline on 6th October). Our only chance of seizing control, before catastrophe becomes inevitable, is through rapid, emergency cooling intervention. We owe it to the young people of today that we grasp the nettle and prepare for solar geoengineering: injecting sulphur dioxide into the stratosphere to mimic the cooling action of major volcanic eruptions. Experimental injection could even start next year: injection anywhere between 45N and 65N would safely limit the lifetime of the aerosol produced to a few months according to climate models. The risks from rapid full-scale intervention could prove tiny in comparison with the risk of leaving intervention too late to prevent catastrophic climate change for much of the world. To boot, a variety of appropriate interventions, together with a drastic cut in greenhouse gas emissions, could restore the planet to a safe, sustainable, biodiverse and productive state within the lifetimes of our young people. |
|
|
|
|
NOAC Meeting points - 11th July 2022
The whole transcript plus meeting notes is here:
https://docs.google.com/document/d/1yVnVuCvfALGi8nT0QG3qEjiurN-eZRug5DFEzikDZkc/edit?usp=sharing
Robert: There is a false orthodoxy that nothing can be done to avoid 1.5 C. Albedo enhancement appears to be taboo or doesn’t exist. There is no serious policy engagement. Climate complications (like medical complications) might be avoided with SRM. It’s ignored because of the moral hazard argument that demonises the fossil fuel (FF) industry. The only thing that could now prevent biodiversity loss, sea level rise (SLR), extreme weather events etc is to increase albedo. Saying otherwise has no scientific basis.
[Albedo enhancement also means Solar Radiation Management – SRM, e.g. Stratospheric Aerosol Injection - SAI, Marine Cloud Brightening – MCB]
Aria: Climate denial to avoid the necessary technology changes is pretty evil. It has already caused millions of deaths.
Robert: Committed warming was in place even during the 1990s.
Aria: It’s important not to oppose reducing emissions.
Robert: Cutting emissions is marginal to stabilising the climate. Too much focus on emissions reduction detracts from what would have more effect. Even greenhouse gas removal (GGR) should not be the primary response.
Aria: We need an ‘all of the above’ approach, and not be adversarial. People should continue operating in their area of expertise. We want to build alliances and not risk masking underlying problems.
Brian: The public need to be brought on board step-by-step that decarbonization is necessary but not sufficient, and planetary albedo needs to be restored. Eg Arctic albedo has reduced from 0.8 to 0.2.
The fossil fuel industry lied for decades, but today they’re not all the same. E.g. Shell has committed to reducing scope 3 (customer) emissions by 45% by 2035. FF and mining companies are among the few with the cash to scale huge technology transformations. That should continue, with maybe 10% of resources going to cooling interventions to avoid the worst effects of warming this century as we get back to a healthy CO2 level, which will take longer.
Robert: Yes, decarbonisation investment should continue, but the priority should move to albedo restoration to avoid the current big risk of Arctic collapse. The public is unaware of the inadequacy of decarbonization.
Brian: Albedo contains many components, but we can say: “Make the planet brighter – and smarter.â€
Aria: Yes, I love that framing.
Sev: Brightening is much less painful than emissions reduction. Priorities need reordering: 1. Brightening, 2. GGR, 3. Emissions reduction, 4. Thermal management, (e.g. cirrus cloud thinning), 5. Adaptation - sea walls are only temporarily effective.
Me: Polarisation is less problematic than demonization. (Prof Kotkin)
Robert: Fossil fuels have delivered high standards of living. It’s risky to reduce those. Demonization hampers civilised dialogue.
Sev: You catch more flies with honey than vinegar.
Aria: But pollution is damaging to human health. Energy efficiency would ameliorate that.
Brian: We could be at the start of a microbial methane bomb. An atmospheric methane depletion intervention could cut the warming rate in half. Perhaps focus 80% of efforts on that, and rebrightening.
Robert: Increasing Arctic albedo is the best way to slow permafrost methane emissions and curb the accelerating feedback loops.
Ron: I’m trying to compile a document of cooling technologies. 1.2 billion people live in countries that depend on fossil fuel earnings, and the Republican Party has been bought by the FF industry.
Bru: Large environmental groups have insufficient understanding of the problems. Closing down the global economy would cause untold suffering. Planetary brightening is an absolute necessity, as is reducing emissions as fast as we can. Our job is to educate the public, while kee |  (Click HERE to view the transcrript) (Click HERE to view the transcrript) |
|
|
|
| 3 |
nature.com/articles/s4159... |
An earth system model shows self-sustained thawing of permafrost even if all man-made GHG emissions stop in 2020 |
11/12/20 |
11/8/23 |
|
|
|
| 3 |
nature.com/articles/s4159... |
An earth system model shows self-sustained thawing of permafrost even if all man-made GHG emissions stop in 2020 |
11/12/20 |
11/8/23 |
|
| Description | The risk of points-of-no-return, which, once surpassed lock the world into new dynamics, have been discussed for decades. Recently, there have been warnings that some of these tipping points are coming closer and are too dangerous to be disregarded. In this paper we report that in the ESCIMO climate model the world is already past a point-of-no-return for global warming. In ESCIMO we observe self-sustained thawing of the permafrost for hundreds of years, even if global society stops all emissions of man-made GHGs immediately. We encourage other model builders to explore our discovery in their (bigger) models, and report on their findings. The thawing (in ESCIMO) is the result of a continuing self-sustained rise in the global temperature. This warming is the combined effect of three physical processes: (1) declining surface albedo (driven by melting of the Arctic ice cover), (2) increasing amounts of water vapour in the atmosphere (driven by higher temperatures), and (3) changes in the concentrations of the GHG in the atmosphere (driven by the absorption of CO2 in biomass and oceans, and emission of carbon (CH4 and CO2) from thawing permafrost). This self-sustained, in the sense of no further GHG emissions, thawing process (in ESCIMO) is a causally determined, physical process that evolves over time. It starts with the man-made warming up to the 1950s, leading to a rise in the amount of water vapour in the atmosphere--further lifting the temperature, causing increasing release of carbon from thawing permafrost, and simultaneously a decline in the surface albedo as the ice and snow covers melts. To stop the self-sustained warming in ESCIMO, enormous amounts of CO2 have to be extracted from the atmosphere. |
| Contents | The possibility of points-of-no-return in the climate system has been discussed for two decades1,2,3. A point-of-no-return can be seen as a threshold which, once surpassed, fundamentally changes the dynamics of the climate system. For example, by triggering irreversible processes like thawing of the permafrost, drying of the rainforests, or acidification of surface waters. Recently, Lenton et al.4 summarized the global situation and warned that thresholds may be closer in time than commonly believed. The purpose of this article is to report that we have identified a point-of-no-return in our climate model ESCIMO - and that it is already behind us. ESCIMO is a "reduced complexity earth system" climate model5 which we run from 1850 to 2500. In ESCIMO the global temperature keeps rising to 2500 and beyond, irrespective of how fast humanity cuts the emissions of man-made greenhouse gas (GHG) emissions. The reason is a cycle of self-sustained thawing of the permafrost (caused by methane release), lower surface albedo (caused by melting ice and snow) and higher atmospheric humidity (caused by higher temperatures). This cycle appears to be triggered by global warming of a mere?+?0.5 °C above the pre-industrial level. |
|
|
|
|
|
| | # |
Name
(N/A = Not Available) |
Title |
Ref Date |
Date Added |
|---|
|
|
|
| Description | West Antarctica is headed for decades of rapid melting no matter how quickly humans cut greenhouse gas emissions, and 2023 shattered records for missing sea ice around the continent. |
| Contents | A trio of new scientific analyses about the loss of ice in Antarctica paint a picture of a continent in trouble. Sea ice is disappearing, gigantic portions of the West Antarctic ice sheet are crumbling and even relatively stable East Antarctica is showing worrying changes. |
|
|
|
|
| Description | The intensifying impacts of climate change are exceeding projections and amplifying the risk of catastrophic harm to the environment and society throughout the 21st century. Planned and proposed rates of emissions reduction and removal are not proceed |
| Contents | The intensifying impacts of climate change are exceeding projections and amplifying the risk of catastrophic harm to the environment and society throughout the 21st century. Planned and proposed rates of emissions reduction and removal are not proceeding at a pace or magnitude to meet either the 1.5°C or 2.0°C targets of the Paris Agreement. Moreover, the impacts, damage and loss occurring at today's 1.2°C of global warming are already significantly disrupting the environment and society. Relying exclusively on greenhouse gas (GHG) emissions reduction and removal without including climate cooling options is thus proving incompatible with responsible planetary stewardship. Multiple approaches to exerting a cooling influence have the potential to contribute to offset at least some of the projected climate disruption if deployed in the near term. Employed thoughtfully, such approaches could be used to limit global warming to well below 1° C, a level that has led to large reductions in sea ice, destabilization of ice sheets, loss of biodiversity, and transformation of ecosystems. An effective plan for avoiding |
|
|
|
| 3 |
zenodo.org/records/8408608 |
FROZEN ARCTIC. A compendium of interventions to slow down, halt, and reverse the effects of climate change in the Arctic and northern regions |
10/4/23 |
10/24/23 |
|
|
|
| 3 |
zenodo.org/records/8408608 |
FROZEN ARCTIC. A compendium of interventions to slow down, halt, and reverse the effects of climate change in the Arctic and northern regions |
10/4/23 |
10/24/23 |
|
| Description | Phase I of the Frozen Arctic Conservation project was a literature review to identify and and document the range of interventions that have been proposed to reverse, stabilize, or delay climate change impacts in the northern and Arctic regions. A total of 61 interventions were identified in six categories: ice sheets and glaciers, sea ice and icebergs, atmosphere and radiation management, marine measures, land-based measures, and industry. The interventions were evaluated according to a set of 12 criteria: technological readiness level, scalability, timeliness for near future effects, potential to make a difference in Arctic and northern regions given enough time, potential to make a global difference given enough time, cost to benefit comparison, likelihood of environmental risks, effects on Indigenous/local communities, ease of reversibility, and likelihood of termination shock. the aim is to follow up this initial evaluation with in-depth analyses of the most promising schemes according to clear, understandable, bias-free, and comparable metrics, including from a right-based approach. |
|
|
|
|
Agenda
Chris GESAMP Report Press Release from IMO
BBC Report Big Seaweed Farm Sargassum South Atlantic
Sev - Trichodesmium cyanobacterium
Cost comparison brightening planet v cutting emissions
COP27 "“ Ocean Pavilion
Concert
From Chris Vivian to Everyone: 07:09 AM
IMP Press Release - https://www.imo.org/en/MediaCentre/PressBriefings/pages/Marine-geoengineering.aspx
From mycomputer to Everyone: 07:09 AM
https://en.wikipedia.org/wiki/Trichodesmium
From Chris Vivian to Everyone: 07:09 AM
BBC News story about Seafields https://www.bbc.co.uk/news/science-environment-63200589
From Me to Everyone: 07:30 AM
https://oceanvisions.org/our-programs/macroalgaeresearchframework/
From Chris Vivian to Everyone: 07:31 AM
https://doi.org/10.1038/nature09950
From Chris Vivian to Everyone: 07:53 AM
Ocean deserts are growing - https://doi.org/10.1038/news.2008.795
Regional Geoengineering Using Tiny Glass Bubbles Would Accelerate the Loss of Arctic Sea Ice - https://doi.org/10.1029/2022EF002815
From Me to Everyone: 08:00 AM
https://oilprice.com/Energy/Energy-General/Chevron-CEO-Blames-Climate-Policies-For-Global-Energy-Crisis.html
From Chris Vivian to Everyone: 08:03 AM
You might be interested in this post - Climate scientists: concept of net zero is a dangerous trap - https://theconversation.com/climate-scientists-concept-of-net-zero-is-a-dangerous-trap-157368
http://newenergytimes.com/v2/sr/companies/RussGeorge/2013/20140224HSRC-vs-Russ-George-counterclaim.pdf
http://newenergytimes.com/v2/sr/companies/RussGeorge/Russ-George-Low-Energy-Nuclear-Reaction-Research-LENR-and-Plankton-Carbon-Credit.shtml |  (Click HERE to view the transcrript) (Click HERE to view the transcrript) |
|
|
|
|
Special guests, Stephen Salter and Peter Wadhams, join Paul Beckwith and Regina Valdez to discuss Marine Cloud Brightening. Stephen Salter is one of the leading voices of the Marine Cloud Brightening (MCB) movement.
As average global temperatures rise, increasing the reflectivity of clouds over the ocean has been studied as a geoengineering method to reflect more solar radiation away from the Earth thus reducing and reversing the warming caused by excess CO2 concentration in the atmosphere.
This CEF program was recorded at COP26, Glasgow, Scotland, in the Durdle Door press conference room on November 4th, 2021, and published on December 13th, 2021. This presentation is brought to you through a collaboration with FacingFuture.TV https://www.youtube.com/c/FacingFuture.
Topics discussed include the following:
- Why some clouds, such as cumulonimbus are dark, and some are light
- Using nozzles installed on ships to spray very small drops of filtered seawater 0.8 microns in diameter.
- Ships spraying filtered droplets of sea water could be deployed to reverse sea level rise and/or save the Arctic sea ice.
- Governments of countries could decide on targets for sea-surface temperatures
- Cost estimates to deploy a fleet of 800 spray vessels/ships
- Advantages of MCB as a geoengineering technique to prevent the worst extremes of climate change
- How we can change the reflectivity of clouds in areas that will most benefit the Earth.
Links:
Earth is dimming due to climate change
https://news.agu.org/press-release/earth-is-dimming-due-to-climate-change/
Special Guests:
Stephen Salter - Emeritus Professor of Engineering Design at the University of Edinburgh and is responsible for creating the concept of the mechanical enhancement of clouds to increase their reflectivity. Professor Salter is a Member of the Order of the British Empire.
Dr. Peter Wadhams - ScD, is emeritus professor of Ocean Physics, and Head of the Polar Ocean Physics Group in the Department of Applied Mathematics and Theoretical Physics, University of Cambridge. He is best known for his work on sea ice.
Regular Panelists:
Paul Beckwith
- Climate Systems Scientist. Professor at the University of Ottawa in the Paleoclimatology Laboratory as well as at Carleton University
Regina Valdez
- Program Director, Climate Reality Project, NYC. GreenFaith Fellow and LEED Green Associate
Video Production:
UNFCCC
COP26 Media Crew in the Durdle Door Press Conference Room
Charles Gregoire
- Electrical Engineer, Webmaster and IT prime for FacingFuture.Earth \u0026 the Climate Emergency Forum; Climate Reality Leader
Heidi Brault
- Video production and website assistant, Organizer and convener, Metadata technician, BA (Psychology), COP26 team lead for FacingFuture.Earth and the Climate Emergency Forum; Climate Reality Leader
Thanks to the following organizations for making this program possible:
- Buddhist Tzu-Chi Foundation
- Interfaith Center for Sustainable Development
- International Society of Ecological Economics
Attributions
Background Music:
- Title: Through the City II
- Author: Crowander
- Source: Free Music Archive
- License: CC BY-NC 4.0
Image and Video: https://climateemergencyforum.org/assets/attributions/2021-12-13-marine-cloud-brightening.html |  (Click HERE to view the transcrript) (Click HERE to view the transcrript) |
|
|
|
|
|
| | # |
Name
(N/A = Not Available) |
Title |
Ref Date |
Date Added |
|---|
|
|
| 1 |
nature.com/articles/s4159... |
An earth system model shows self-sustained thawing of permafrost even if all man-made GHG emissions stop in 2020 |
11/12/20 |
11/8/23 |
|
|
|
| 1 |
nature.com/articles/s4159... |
An earth system model shows self-sustained thawing of permafrost even if all man-made GHG emissions stop in 2020 |
11/12/20 |
11/8/23 |
|
| Description | The risk of points-of-no-return, which, once surpassed lock the world into new dynamics, have been discussed for decades. Recently, there have been warnings that some of these tipping points are coming closer and are too dangerous to be disregarded. In this paper we report that in the ESCIMO climate model the world is already past a point-of-no-return for global warming. In ESCIMO we observe self-sustained thawing of the permafrost for hundreds of years, even if global society stops all emissions of man-made GHGs immediately. We encourage other model builders to explore our discovery in their (bigger) models, and report on their findings. The thawing (in ESCIMO) is the result of a continuing self-sustained rise in the global temperature. This warming is the combined effect of three physical processes: (1) declining surface albedo (driven by melting of the Arctic ice cover), (2) increasing amounts of water vapour in the atmosphere (driven by higher temperatures), and (3) changes in the concentrations of the GHG in the atmosphere (driven by the absorption of CO2 in biomass and oceans, and emission of carbon (CH4 and CO2) from thawing permafrost). This self-sustained, in the sense of no further GHG emissions, thawing process (in ESCIMO) is a causally determined, physical process that evolves over time. It starts with the man-made warming up to the 1950s, leading to a rise in the amount of water vapour in the atmosphere--further lifting the temperature, causing increasing release of carbon from thawing permafrost, and simultaneously a decline in the surface albedo as the ice and snow covers melts. To stop the self-sustained warming in ESCIMO, enormous amounts of CO2 have to be extracted from the atmosphere. |
| Contents | The possibility of points-of-no-return in the climate system has been discussed for two decades1,2,3. A point-of-no-return can be seen as a threshold which, once surpassed, fundamentally changes the dynamics of the climate system. For example, by triggering irreversible processes like thawing of the permafrost, drying of the rainforests, or acidification of surface waters. Recently, Lenton et al.4 summarized the global situation and warned that thresholds may be closer in time than commonly believed. The purpose of this article is to report that we have identified a point-of-no-return in our climate model ESCIMO - and that it is already behind us. ESCIMO is a "reduced complexity earth system" climate model5 which we run from 1850 to 2500. In ESCIMO the global temperature keeps rising to 2500 and beyond, irrespective of how fast humanity cuts the emissions of man-made greenhouse gas (GHG) emissions. The reason is a cycle of self-sustained thawing of the permafrost (caused by methane release), lower surface albedo (caused by melting ice and snow) and higher atmospheric humidity (caused by higher temperatures). This cycle appears to be triggered by global warming of a mere?+?0.5 °C above the pre-industrial level. |
|
|
|
|
|
| | # |
Name
(N/A = Not Available) |
Title |
Ref Date |
Date Added |
|---|
|
|
|
| Description | Earth is a cool place
Earth is home to nearly 9 million species of plants and animals, 8 billion humans and surely the most beautiful and diverse nature in the known universe. We can be proud of our incredible planet.
Our planet is in need
Earth is under threat. Global temperatures have risen by around 1.2 degrees since the industrial revolution; the consequences of this warming are already dramatic, but they are minor in comparison to what lies ahead. |
| Contents | The Planet Earth Coolkit
It’s time to act in order to keep the planet cool. Humans can help Earth turn down its thermostat, enhancing its own natural processes to reduce global temperatures. We have grouped these processes together into what we call The Planet Earth Coolkit.
We need you to help us get the message out that global cooling is possible!
Global warming is the biggest threat to mankind. It is time now to Cool Planet Earth. |
|
|
|
|
| Author | Peter Fiekowsky and Carole Douglis |
| Description | Costs determine scalability, and costs vary by a factor of 30,000 |
| Contents | One purpose of CDR is to provide “offsets” that legitimize continuing emissions.
The other is to restore the pre-industrial climate by 2050 by removing legacy CO2.
Carbon-dioxide removal (CDR)) tops the tech headlines with increasing frequency, leading to the impression that we are rapidly developing a multitude of mostly industrial options for creating a safe climate.
What many miss is that the vast majority of “carbontech” CDR approaches are designed to develop the carbon-offset market. By definition, offsets only compensate for continued emissions. They do not touch the 1,000 gigatons of legacy CO2 that is causing most of the climate havoc.
In fact, CDR today serves two distinct policy purposes. Each has merit, yet achieving the two goals requires quite different approaches and budgets and would create strikingly different results.
The two goals of CDR are:
Developing a CDR industry that underpins the carbon-offset market—thus adhering literally to the 1992 United Nations goal to “stabilize” greenhouse gas levels. Today this means stabilizing at dangerous levels never before experienced by our species. This is of course now called “net-zero emissions;” and
Following what appears to be the original intent of the United Nations Framework Convention on Climate Change: to restore GHG levels proven safe for humanity and nature as we know it. Restoring historically safe GHG levels is commonly called “climate restoration.”
|
|
|
|
|
| Description | Climate catalyst, the main aerosol described herein, is designed to remove methane and other powerful warming pollutants from the air, adding to its cooling effect. |
| Contents | In this paper we propose that as air pollution is cleaned up, the lost cloud reflectivity could be safely replaced and further increased with benign aerosols. The effect would be to cool the world's oceans and restore more clement weather conditions. One study has suggested that carefully targeted increases of cloud reflectivity could not only restore rainfall patterns but further improve them (Ref Norwegian study).
Climate catalyst, the main aerosol described herein, is additionally designed to remove methane and other powerful warming pollutants from the air, adding to its cooling effect. However, even if only cloud reflectivity were to be increased at sufficient scale in the appropriate places, the effect would be an immediate reversal of the current warming trend. That would buy more time for the important goal of Net Zero emissions to be achieved globally. In the meantime, it would also save countless lives, infrastructure, money, ecosystems, and species from extinction. However, even if only cloud reflectivity were to be increased at sufficient scale in the appropriate places, the effect would be an immediate reversal of the current warming trend. That would buy more time for the important goal of Net Zero emissions to be achieved globally. In the meantime, it would also save countless lives, infrastructure, money, ecosystems, and species from extinction. |
|
|
|
| 4 |
X-FJvzgrM00 (17) |
The money men know the truth about planetary boundaries! |
10/29/23 |
11/13/23 |
|
|
|
| 4 |
X-FJvzgrM00 (17) |
The money men know the truth about planetary boundaries! |
10/29/23 |
11/13/23 |
|
Scientists have identified nine so-called 'Earth System boundaries' beyond which life on our planet will become extremely difficult for many species, not least us humans. That analysis has often been met with scepticism, but risk managers at the world's largest financial institutions have been watching the rapid 'real-world' changes in earth's atmosphere and the catastrophic impacts on their asset portfolios, and they're beginning to factor 'Planetary Boundary' science into their spreadsheets. And when the 'money-men' change, the whole world changes!!
Help support this channels independence at
http://www.patreon.com/justhaveathink
Or with a donation via Paypal by clicking here
https://www.paypal.com/cgi-bin/webscr?cmd=_s-xclick\u0026hosted_button_id=GWR73EHXGJMAE\u0026source=url
You can also help keep my brain ticking over during the long hours of research and editing via the nice folks at BuyMeACoffee.com
https://www.buymeacoffee.com/justhaveathink
Video Transcripts available at our website
http://www.justhaveathink.com
Research Links
The Emperors New Climate Scenarios - Main Paper
https://actuaries.org.uk/media/qeydewmk/the-emperor-s-new-climate-scenarios.pdf
A Safe Operating Space for Humanity - Rockström et al
https://www.nature.com/articles/461472a
Earth beyond six of nine planetary boundaries - Richardson et el
https://www.science.org/doi/pdf/10.1126/sciadv.adh2458?trk=public_post_comment-text
https://www.scientificamerican.com/article/humans-have-crossed-6-of-9-planetary-boundaries/
Climate Central - flood maps
https://coastal.climatecentral.org/
Global Carbon Project
https://www.globalcarbonproject.org/carbonbudget/22/files/GCP_CarbonBudget_2022.pdf
Carbon Tracker
https://carbontracker.org/
IPCC AR6 Report
https://www.ipcc.ch/assessment-report/ar6/
Climate Crisis Advisory Group paper
https://static1.squarespace.com/static/60ccae658553d102459d11ed/t/6253ff0eb27d617aac93cde0/1649671961939/CCAG_PositionPaper_CriticalPathway.pdf
Chatham House article
https://www.chathamhouse.org/2023/07/climate-change-threatens-cause-next-economic-mega-shock
'The Conversation' article
https://theconversation.com/climate-change-could-lead-to-food-related-civil-unrest-in-uk-within-50-years-say-experts-214754
Check out other YouTube Climate Communicators
zentouro: https://www.youtube.com/user/zentouro
Climate Adam: https://www.youtube.com/user/ClimateAdam
Kurtis Baute: https://www.youtube.com/user/ScopeofScience
Levi Hildebrand: https://www.youtube.com/user/The100LH
Simon Clark: https://www.youtube.com/user/SimonOxfPhys
Sarah Karvner: https://www.youtube.com/channel/UCRwMkTu8sCwOOD6_7QYrZnw
Rollie Williams / ClimateTown: https://www.youtube.com/channel/UCuVLG9pThvBABcYCm7pkNkA
Jack Harries: https://www.youtube.com/user/JacksGap
Beckisphere: https://www.youtube.com/channel/UCT39HQq5eDKonaUV8ujiBCQ
Our Changing Climate : https://www.youtube.com/channel/UCNXvxXpDJXp-mZu3pFMzYHQ
Engineering With Rosie https://www.youtube.com/c/EngineeringwithRosie
Ella Gilbert https://www.youtube.com/c/DrGilbz
Planet Proof https://www.youtube.com/channel/UCdtF58iBRQ2C3QPeKKzxwiA
Our Eden https://www.youtube.com/@OurEden |  (Click HERE to view the transcrript) (Click HERE to view the transcrript) |
|
|
|
| 5 |
apple.news/AmxD0XtrIR-SAi... |
Scientists sound alarm after research finds new danger for tropical trees: 'The tip of the iceberg in terms of effects' |
9/29/23 |
11/8/23 |
|
|
|
| 5 |
apple.news/AmxD0XtrIR-SAi... |
Scientists sound alarm after research finds new danger for tropical trees: 'The tip of the iceberg in terms of effects' |
9/29/23 |
11/8/23 |
|
| Description | Trees in tropical forests may soon reach a critical temperature at which they begin to fail to take in carbon dioxide. |
| Contents | “There could be massive leaf death, possible tree mortality, and species turnover across all tropical forests.” |
|
|
|
|
|
|
|
|
| | # |
Name
(N/A = Not Available) |
Title |
Ref Date |
Date Added |
|---|
|
|
|
| Description | This is the second article in a two-part series. Read the first part here.
Climate model scenarios similar to current policies project 2°C of warming before 2050; if James Hansen is right (see Part 1) and warming sharply accelerates, it could be a decade sooner. These outcomes will be driven by the high energy imbalance, continuing high emissions, the accelerating accumulation of heat in the oceans, and decreases in short-term aerosol cooling. |
| Contents | Several years ago a group of eminent scientists proposed a “carbon law”, which said that keeping warming to 2°C required emissions to be halved every decade from 2020 onwards, including a halving between 2020 and 2030, plus some carbon drawdown. Instead, the level of greenhouse gases and coal use both hit record highs in 2023. And the largest national fossil fuel producers plan to keep on expanding production As a result, current government plans worldwide will likely result in emissions in 2050 almost as high as they are today, according to the UN Environment Programme’s 2023 Production Gap report.
Other analyses are broadly consistent:
The International Energy Agency finds that stated national policies will result in oil and gas production in 2050 as high as 2020; with coal halved.
The OECD finds that a world economy four times larger than today is projected to need 80% more energy in 2050; and without new policy action and the global energy mix in 2050 will not differ significantly from today.
The intentions of the world’s five largest fossil fuel producers are clear — and civilisation-threatening — as reported by the UN:
In China, oil production is projected to be flat to 2050, but gas will increase more than 60 percent from 2020 to 2050, while coal use will remain high till 2030 then decline sharply.
In the United States, oil production will grow and then remain at record levels to 2050, and gas is projected to continuously and significantly increase to 2050; whilst coal will drop by half.
Projections for Russia are available only to 2035, with coal and gas production projected to increase significantly, while oil remains flat.
In Saudi Arabia, oil production is projected to grow by 26 to 47 percent by 2050, with gas up 40 percent between 2019 and 2050. Together they make up half of the Saudi economy.
And in Australia, one of the world’s top two liquified natural gas and coal exporters, gas production is projected to stay above the current level for the next 15 years, with coal remaining high over the same period, above 450 million metric tons annually.
We are heading towards 3–4°C.
This outlook suggests Earth is heading towards 3°C of warming and perhaps a good deal more, because current climate models which project warming of around 2.7°C do not adequately account for all the system-level reinforcing feedbacks.
In 2021, the pre-eminent UK international affairs think-tank Chatham House said a “plausible worst-case scenario” is 3.5°C or more, which could be an underestimate if tipping points are reached sooner than the orthodox science suggests. This now seems to be the reality.
A clear majority of scientists expected warming of more than 3°C, and 82% expected to see catastrophic impacts of climate change in their lifetime, according to a 2021 survey by the journal Nature.
Questions about the size of the aerosol forcing, and the related issue of how sensitive the climate is to changes in greenhouse gases, remain an issue of scientific contention.
New climate history research published in December 2023, based on a study of the last 66 million years, concluded that global temperature may be more sensitive to CO2 levels than current models estimate. It showed that the last time CO2 levels were as high as today was around 14 million year ago, which is longer than previous estimates, and that climate sensitivity — the amount of warming resulting from a doubling of atmospheric CO2 — may be between between 5°C and 8°C, compared to the IPCC orthodoxy of 1.5–4.5°C.
The level of greenhouse gases is currently around 560 parts per million, double the pre-industrial level. Some of those gases such as methane are short-lived so this level of forcing is not written in stone, but nevertheless if Hansen et al. are right that a doubling may lead to around 4–5°C of warming, then another 30 years of high emissions means humans will have created an increasingly unliveable planet.
Has the impact of aerosols been widely understood? In what the New York Times described as “an eye-opening Nature commentary”, Geeta Persad and her colleagues wrote in late 2022 that “overall, vast emissions of aerosols since the start of the industrial age have had a profound cooling effect” and that without them “the global warming we see today would be 30 to 50 percent greater”, warning that “the impacts of aerosols on climate risk are often ignored”.
In 2018, a group of eminent scientists explored the potential — once warming had exceeded the 1.5–2°C range — for self-reinforcing positive feedbacks in major elements of the climate system to push passed a planetary threshold that would prevent temperature stabilisation, and drive the system to a “Hothouse Earth”. They warned that “we are in a climate emergency… this is an existential threat to civilisation”.
The 2023 State of the Climate Report: Entering uncharted territory warned of: “potential collapse of natural and socioeconomic systems in such a world [of 2.6°C warming] where we will face unbearable heat, frequent extreme weather events, food and fresh water shortages, rising seas, more emerging diseases, and increased social unrest and geopolitical conflict.”
Whatever the words, the understanding is widely shared that contemporary nations and societies, and likely the global social system, are heading towards collapse. “If we carry on the way we are going now, I can’t see this civilisation lasting to the end of this century”, says Professor Tim Lenton. The US Defence Secretary Lloyd Austin III calls the risks “existential”.
Opening the Innovation Zero Congress in London in May 2023, Potsdam Institute Director Prof. Johan Rockstrom described the path we are on:
“2.5°C global mean surface temperature rise is a disaster. It’s something that humanity has absolutely no evidence that we can cope with… [There] would be a 10-metre sea-level rise. There would be a collapse of all the big biomes on planet Earth – the rainforest, many of the temperate forests – abrupt thawing of permafrost, we will have complete collapse of marine biology… Over one-third of the planet around the equatorial regions will be uninhabitable because you will pass the threshold of health, which is around 30°C. It’s only in some parts of the Sahara Desert today that has that kind of average temperature.”
Chatham House’s Climate Risk Assessment 2021 concludes that by 2050 global food demand would be 50% higher, but crop yields may drop by 30%. As desertification spreads across the dry sub-tropics, and one-third of the planet experiences unprecedented heat, it is not difficult to see why they concluded that cascading climate impacts will “drive political instability and greater national insecurity, and fuel regional and international conflict”.
What is worse is the setback to climate action posed by current conflicts and military posturing in Europe, the Middle East and east Asia, which are huge political distractions from dealing with the greatest threat to humanity, and all of which have the potential to spread more widely.
To maintain military flexibility, the US insisted in 1997 that direct military carbon emissions be excluded from international carbon accounting. Those emissions, around 5 percent of the total global, are far less than the indirect emissions from conflict, as recent estimates here and here indicate.
Projections show that by 2100 the expansion of the Sahara due to desertification will embrace Israel/Palestine, as well as spreading across the Mediterranean into Spain, Italy, Greece and Turkey (see map).
The Australian Prime Minister has finally spoken out about the escalating climate threat whilst inspecting damage from the recent Queensland floods: “All of this is a reminder that the science told us that climate change would mean there would be more extreme weather events and they would be more intense. And unfortunately, we are seeing that play out with the number of events that we’re having to deal with right around Australia”.
Just so, except that in common with leaders globally, the Australian government continues to have its head stuck in the sand about the real risks climate change now represents. It refuses to release an intelligence assessment of climate-security risks, and has fumbled a domestic climate risk assessment.
As a result, the community remains ill-informed and unprepared for what is coming. |
|
|
|
|
| Description | Climatic Change - |
| Contents | "Moral hazard" links geoengineering to mitigation via the fear that either solar geoengineering (solar radiation management, SRM) or carbon dioxide removal (CDR) might crowd out the desire to cut emissions. Fear of this crowding-out effect ranks among the most frequently cited risks of (solar) geoengineering. We here test moral hazard versus its inverse in a large-scale, revealed-preference experiment (n?~?340,000) on Facebook and find little to no support for either outcome. For the most part, talking about SRM or CDR does not motivate our study population to support a large US environmental non-profit's mission, nor does it turn them off relative to baseline climate messaging, except when using extreme messengers and framings. Our results indicate the importance of actors and reasoned narratives of (solar) geoengineering to help guide public discourse. |
|
|
|
|
| Description | Yale Climate Connections is a news service that aims to help you understand the reality of climate change and what you can do about it. Through our website, YouTube channel, and national radio program, which airs each day on hundreds of stations, we reach millions of people like you each year.
|
| Contents | We are staffed by professional journalists, meteorologists, and radio producers. We’re independent and nonpartisan. Yale Climate Connections is an initiative of the Yale Center for Environmental Communication, directed by Dr. Anthony Leiserowitz of the Yale School of the Environment at Yale University.
Most content is published under Creative Commons: Attribution-Noncommercial-No Derivative Works. Please visit our Use & Privacy Policy page for additional information.
Yale Climate Connections is grateful for the generous financial support of the Grantham Foundation for the Protection of the Environment and of individual Yale University alumni. We also thank the CO2 Foundation for its support of our Spanish-language articles. Yale Climate Connections is solely responsible for all content. |
|
|
|
|
| Description | There is clear scientific consensus that carbon
dioxide removal (CDR) — alongside a strong
prioritization of greenhouse gas emissions
reduction — will be required at an immense,
multi-gigatonne (Gt) annual scale by mid-century
to limit warming to 1.5 or even 2°C.1
Covering 71%
of the planet’s surface, the ocean has served as a
critically important sink for anthropogenic carbon
dioxide (CO2), absorbing over 25% of annual
emissions.2
The ocean has also absorbed about
90% of the heat that has accumulated in the Earth
system due to rising atmospheric CO2.
3
The ocean
thus provides a vital climate mitigation function,
but this has come at significant, and increasing,
cost to ocean health, marine ecosystems, and
biodiversity |
| Contents | Given its enormous scale, the ocean has an
outsized potential role to play in advancing CDR
to the level required to meet Paris Agreement
targets. Marine CDR (mCDR) has the potential,
when responsibly deployed and scaled, to
offer significant climate benefits, while also
contributing to sustainable economic development
for coastal communities, maritime nations, and
small island developing states (SIDS). Additionally,
certain mCDR approaches may yield co-benefits
such as improvements to ocean health, via local
mitigation of ocean acidification, to coastal
ecosystems and commercial aquaculture.
Examples of mCDR include:
Ocean alkalinity enhancement (OAE) via
electrochemical systems, or the physical
application of clean alkaline minerals to
coastlines, coastal watersheds, or the open ocean;
Electrochemical or photochemical systems that
directly remove CO2 from the ocean;
Dedicated cultivation or harvesting of aquatic
biomass, including macroalgae and microalgae (for
sinking to the deep ocean, long-duration terrestrial
storage, or potential incorporation into long-lived
products); and
Restoration, enhancement, and scaling of carbon
sinks associated with seagrass, mangroves, and
other coastal marine ecosystems (coastal “blue
carbon”). |
|
|
|
|
| Description | Global heating of the Earth system is unequivocal. However, detecting an acceleration of Earth heating has remained elusive to date, despite suggestive evidence of a potential increase in heating rates. In this study, we demonstrate that since 1960, the warming of the world ocean has accelerated at a relatively consistent pace of 0.15 ± 0.05 (W/m2)/decade, while the land, cryosphere, and atmosphere have exhibited an accelerated pace of 0.013 ± 0.003 (W/m2)/decade. This has led to a substantial increase in ocean warming, with a magnitude of 0.91 ± 0.80 W/m2 between the decades 1960-1970 and 2010-2020, which overlies substantial decadal-scale variability in ocean warming of up to 0.6 W/m2. Our findings withstand a wide range of sensitivity analyses and are consistent across different observation-based datasets. The long-term acceleration of Earth warming aligns qualitatively with the rise in CO2 concentrations and the decline in aerosol concentration during the same period, but further investigations are necessary to properly attribute these changes. |
| Contents | In the past 150 years, Earth's climate has been warming at a rate that is unprecedented in at least the last 2000 years1. This human-caused warming has caused widespread adverse impacts and related losses and damages to nature and people, which will continue in the future as global climate continues to warm2. Detecting changes in the rate of warming is crucial for informed decision-making in international climate negotiations, with the aim of limiting global warming to specific levels. However, it remains a significant challenge to detect such changes due to the substantial internal variability of the climate system on a decadal scale (e.g., ref. 3). In this paper, we address this challenge by examining the global heat accumulation rate across the entire climate system, including the ocean, atmosphere, cryosphere, and land. By focusing on this integrated view, rather than solely relying on changes in global mean surface temperature, we can mitigate the impact of variability and gain a more comprehensive understanding4,5. Global heat accumulation in the climate system, resulting from the current positive Earth's Energy Imbalance (EEI) at the top of the atmosphere, is primarily dominated by changes in Global Ocean Heat Content (GOHC)4. GOHC changes account for approximately 90% of the total heat increase in the past fifty years, while land heating, ice melting, and atmospheric warming contribute around 5%, 3%, and 1% respectively6,7,8. Several studies have indicated an increase in the global heat accumulation rate in recent decades, with values rising from 0.50 [0.32 to 0.69] W/m2 during the period 1971-2006 to 0.79 [0.52 to 1.06] W/m2 (90% confidence interval) for the period 2006-2018 (ref.4,6,7,8,9,10,11,12,13,14,15,16,17,18,19,20,21,22 and Fig. 1). Some studies have even suggested a potential doubling of EEI in the last decade compared to the previous one6,17. Figure 1 |
|
|
|
|
|
| | # |
Name
(N/A = Not Available) |
Title |
Ref Date |
Date Added |
|---|
|
|
|
| Description | Human survival depends on this iconic ecosystem, and only one thing will save it. |
| Contents | Human survival depends on this iconic ecosystem, and only one thing will save it. |
|
|
|
|
|
| | # |
Name
(N/A = Not Available) |
Title |
Ref Date |
Date Added |
|---|
|
|
|
| Author | David Spratt and Ian Dunlop |
| Description | For climate change, 2023 was an “unprecedented” year, “absolutely gobsmackingly bananas” and “scary” and “frightening”. And that was what climate scientists said! The UN Secretary General called it the year in which humanity crossed into a new climate era — an age of “global boiling”.
Climate disruption shocked climate scientists in 2023. “Surprising. Astounding. Staggering. Unnerving. Bewildering. Flabbergasting. Disquieting. Gobsmacking. Shocking. Mind boggling,” said Prof. Ed Hawkins when September 2023 exceeded the previous September record by a huge 0.5°C.
The decline in Antarctic sea-ice extent was much greater than model projections, leading the National Snow and Ice Data Centre’s Walt Meier to exclaim: “It’s so far outside anything we’ve seen, it’s almost mind- blowing.”
Many records were set for new climate extremes — record heat, rainfall and floods — with some of it driven by the destabilisation of the polar jet stream. “We are hitting record breaking extremes much sooner than I expected. That’s frightening, scary, and concerning, and it really suggests that we’re not as aware of what’s coming as we thought we were,” said Sarah Perkins-Kirkpatrick of the University of NSW. |
| Contents | With devastating extreme heat and storms and floods, 2023 was the first year 1.5°C warmer than the 1850-1900 baseline, and both Antarctic sea-ice loss and record northern hemisphere sea-surface temperatures were way beyond the ranges projected by climate models.
Datasets of global temperatures vary a little depending on method, but two of the most significant are Berkeley Earth which put 2023 at 1.54°C above the pre-industrial (1850-1900) level, and Copernicus/ECMWF at 1.48°C.
Berkeley said that “a single year exceeding 1.5°C is a stark warning sign of how close the overall climate system has come to exceeding this Paris Agreement goal. With greenhouse gas emissions continuing to set record highs, it is likely that climate will regularly exceed 1.5°C in the next decade.”
2023 was notable for:
Global average warming hitting the 1.5°C mark, and new monthly records for global temperature every month from June to December. The October to December period was 1.74°C.
New national record high annual averages for an estimated 77 countries.
The first year that global average ocean surface temperatures exceeded 1°C, with once-in-a-century levels of warmth in the North Atlantic.
Two days in November when global average temperature, for the first time ever, reached 2°C above the pre-industrial levels.
Catastrophic flooding from Greece to Beijing to Vermont, and earlier in the year major flooding in New Zealand associated with a rain bomb and then cyclone Gabrielle.
Severe wildfires in Europe, Russia, Maui and North America; fires in Canada burned 18.5 million hectares of land.
The 2023 extremes were a shock. Prof. Katharine Hayhoe told the Guardian that: “We have strongly suspected for a while that our projections are underestimating extremes, a suspicion that recent extremes have proven likely to be true… We are truly in uncharted territory in terms of the history of human civilisation on this planet.”
Explanations for 2023 are incomplete, but warming is accelerating and 2024 is likely to be hotter
What happened in 2023 was not what scientists’ models anticipated at the beginning of the year and fell well outside the confidence intervals of any of the estimates. Carbon Brief says that “while there are a number of factors that researchers have proposed to explain 2023’s exceptional warmth, scientists still lack a clear explanation for why global temperatures were so unexpectedly high… researchers are just starting to disentangle the causes of the unexpected extreme global heat the world experienced in 2023”.
One person who has a clear view is the former NASA climate chief James Hansen who says that “the 1.5 degree limit is deader than a doornail” and warns that warming will accelerate to 1.7°C by 2030 and “2°C will be reached by the late 2030s”. |
|
|
|
|
| Description | There is clear scientific consensus that carbon
dioxide removal (CDR) — alongside a strong
prioritization of greenhouse gas emissions
reduction — will be required at an immense,
multi-gigatonne (Gt) annual scale by mid-century
to limit warming to 1.5 or even 2°C.1
Covering 71%
of the planet’s surface, the ocean has served as a
critically important sink for anthropogenic carbon
dioxide (CO2), absorbing over 25% of annual
emissions.2
The ocean has also absorbed about
90% of the heat that has accumulated in the Earth
system due to rising atmospheric CO2.
3
The ocean
thus provides a vital climate mitigation function,
but this has come at significant, and increasing,
cost to ocean health, marine ecosystems, and
biodiversity |
| Contents | Given its enormous scale, the ocean has an
outsized potential role to play in advancing CDR
to the level required to meet Paris Agreement
targets. Marine CDR (mCDR) has the potential,
when responsibly deployed and scaled, to
offer significant climate benefits, while also
contributing to sustainable economic development
for coastal communities, maritime nations, and
small island developing states (SIDS). Additionally,
certain mCDR approaches may yield co-benefits
such as improvements to ocean health, via local
mitigation of ocean acidification, to coastal
ecosystems and commercial aquaculture.
Examples of mCDR include:
Ocean alkalinity enhancement (OAE) via
electrochemical systems, or the physical
application of clean alkaline minerals to
coastlines, coastal watersheds, or the open ocean;
Electrochemical or photochemical systems that
directly remove CO2 from the ocean;
Dedicated cultivation or harvesting of aquatic
biomass, including macroalgae and microalgae (for
sinking to the deep ocean, long-duration terrestrial
storage, or potential incorporation into long-lived
products); and
Restoration, enhancement, and scaling of carbon
sinks associated with seagrass, mangroves, and
other coastal marine ecosystems (coastal “blue
carbon”). |
|
|
|
|
| Author | Graeme Taylor (BEST Futures), Peter Wadhams (University of Cambridge), Daniele Visioni (Cornell University), Tom Goreau (Global Coral Reef Alliance), Leslie Field (Stanford University), Heri Kuswanto (Institut Teknologi Sepuluh Nopember) |
| Description | Although the 2015 Paris Agreement climate targets seem certain to be missed, only a few experts are questioning the adequacy of the current approach to limiting climate change and suggesting that additional approaches are needed to avoid unacceptable catastrophes. This article posits that selective science communication and unrealistically optimistic assumptions are obscuring the reality that greenhouse gas emissions reduction and carbon dioxide removal will not curtail climate change in the 21st Century. It also explains how overly pessimistic and speculative criticisms are behind opposition to considering potential climate cooling interventions1 as a complementary approach for mitigating2 dangerous warming. |
| Contents | There is little evidence supporting assertions that: current greenhouse gas emissions reduction and removal methods can and will be ramped up in time to prevent dangerous climate change; overshoot of Paris Agreement targets will be temporary; net zero emissions will produce a safe, stable climate; the impacts of overshoot can be managed and reversed; Intergovernmental Panel on Climate Change models and assessments capture the full scope of prospective disastrous impacts; and the risks of climate interventions are greater than the risks of inaction.
These largely unsupported presumptions distort risk assessments and discount the urgent need to develop a viable mitigation strategy. Due to political pressures, many critical scientific concerns are ignored or preemptively dismissed in international negotiations. As a result, the present and growing crisis and the level of effort and time that will be required to control and rebalance the climate are severely underestimated.
In conclusion, the paper outlines the key elements of a realistic policy approach that would augment current efforts to constrain dangerous warming by supplementing current mitigation approaches with climate cooling interventions. |
|
|
|
|
Healthy Planet Action Coalition
https://www.healthyplanetaction.org/
Title: Model Simulations of Climate Interventions Aiming to Offset Future Warming: Insights and Uncertainties
Speaker: Associate Professor Douglas MacMartin, Cornell University
Talk Overview
Decadal-average global warming is approaching 1.2 C and it is likely that the 1.5 C goal from the Paris Agreement will be passed in the next decade or so. Global warming is now being experienced through the increasing likelihood of severe weather, more intense storms, destabilization of major glacial streams, increasing rate of rise of sea level, and more, all driven by the ongoing emissions of greenhouse gas emissions.
With the present and projected pace of emissions mitigation, global warming is projected to at least double before net-zero emissions are reached up to a few decades after mid-century, with corresponding increased impacts and risks.
With all nations committed to the goal of keeping global warming to no more than 1.5 C and climate intervention becoming the only option for preventing further warming, modeling studies have started looking at climate intervention scenarios that would offset further warming, stabilizing the climate at 1.5C, or restoring back to 1.0C or lower.
Professor MacMartin reports on the status of climate stabilization studies using stratospheric aerosol injection (SAI), providing an overview of what would be involved, including options such as more polar-focused deployments, what the resulting stabilized climate would be like and how long it might take to reach a desired cooling, what the key uncertainties are and how they might compare to the types of consequences that might trigger calls for intervention, and what research is needed to provide the firmer information needed for early rather than late-stage emergency intervention to be considered as a potential policy scenario.
The recording includes a powerpoint presentation followed by question and answer with the audience.
Biography (from https://www.mae.cornell.edu/faculty-directory/douglas-macmartin
Douglas MacMartin is an Associate Professor in the Sibley School of Mechanical and Aerospace Engineering at Cornell University.
His research focuses on Sunlight Reflection Methods (SRM, also known as climate engineering, solar geoengineering, or climate intervention), with the aim of helping to develop the knowledge base necessary to support informed future societal decisions in this challenging and controversial field.
He has published extensively on the subject, and in addition to public and academic presentations has provided briefings to the UN Environment Program and testimony to the US Congress, and he was a member of the US National Academies panel that made recommendations on both research and governance in March 2021.
Doug received his Ph.D. in Aeronautics and Astronautics from MIT in 1992; previous positions include United Technologies Research Center (1994-2000) and the California Institute of Technology (2000-2015). His research is funded by NSF and by the Cornell Atkinson Center for a Sustainable Future. |  (Click HERE to view the transcrript) (Click HERE to view the transcrript) |
|
|
|
|
| Description | We need a new way of talking about global warming. UN Secretary General António
Guterres underscored this when he said the “era of global boiling” has arrived. Although
we have made remarkable progress on a very complex problem over the past thirty years,
we have a long way to go before we can keep the global temperature increase to below 2°C
relative to the pre-industrial times. Climate models suggest that this next decade is critical
if we are to avert the worst consequences of climate change. The world must continue
to reduce greenhouse gas emissions, and find ways to adapt and build resilience among
vulnerable communities. At the same time, we need to find new ways to remove carbon
dioxide from the atmosphere in order to chart a “net negative” emissions pathway. Given
their large capacity for carbon storage, the oceans must be included in consideration of
our multiple carbon dioxide removal (CDR) options (1).
This report focused on ocean iron fertilization (OIF) for marine CDR. This is by no
means a new scientific endeavor. Several members of ExOIS (Exploring Ocean Iron
Solutions) have been studying this issue for decades, but the emergence of runaway climate
impacts has motivated this group to consider a responsible path forward for marine
CDR. That path needs to ensure that future choices are based upon the best science and
social considerations required to reduce human suffering and counter economic and ecological
losses, while limiting and even reversing the negative impacts that climate change
is already having on the ocean and the rest of the planet.
Prior studies have confirmed that the addition of small amounts of iron in some parts
of the ocean is effective at stimulating phytoplankton growth. Through enhanced photosynthesis,
carbon dioxide can not only be removed from the atmosphere but a fraction
can also be transferred to durable storage in the deep sea. However, prior studies were
not designed to quantify how effective this storage can be, or how wise OIF might be as
a marine CDR approach. |
| Contents | needed to answer critical questions about the potential efficiency and ecological impacts
of marine CDR (http://oceaniron.org). Owing to concerns surrounding the ethics of marine
CDR, ExOIS is organized around a responsible code of conduct that prioritizes activities
for the collective benefit of our planet with an emphasis on open and transparent
studies that include public engagement (2; see inset pg. 3).
Our goal is to establish open-source conventions for implementing OIF for marine
CDR that can be assessed with appropriate monitoring, reporting, and verification
(MRV) protocols, going beyond just carbon accounting, to assess ecological and other
non-carbon environmental effects (eMRV). As urgent as this is, it will still take 5 to 10
years of intensive work and considerable resources to accomplish this goal.
We present here a “Paths Forward’’ report that stems from a week-long workshop held
at the Moss Landing Marine Laboratories in May 2023 that was attended by international
experts spanning atmospheric, oceanographic, and social sciences as well as legal specialists
(see inside back cover). At the workshop, we reviewed prior OIF studies, distilled
the lessons learned, and proposed several paths forward over the next decade to lay the
foundation for evaluating OIF for marine CDR. Our discussion very quickly resulted in
a recommendation for the need to establish multiple “Ocean Iron Observatories’’ where,
through observations and modeling, we would be able to assess with a high degree of
certainty both the durable removal of atmospheric carbon dioxide—which we term the
“centennial tonne”—and the ecological response of the ocean.
3 PATHS FORWARD FOR EXPLORING OCEAN IRON FERTILIZATION
In a five-year phase I period, we prioritize five major research activities:
1. Next generation field studies
Studies of long-term (durable) carbon storage will need to be longer (year or more) and
larger (>10,000 km2) than past experiments, organized around existing tools and models, but
with greater reliance on autonomous platforms. While prior studies suggested that ocean
systems return to ambient conditions once iron infusion is stopped, this needs to be verified.
We suggest that these next field experiments take place in the NE Pacific to assess the
processes controlling carbon removal efficiencies, as well as the intended and unintended
ecological and geochemical consequences.
2. Regional, global and field study modeling
Incorporation of new observations and model intercomparisons are essential to accurately
represent how iron cycling processes regulate OIF effects on marine ecosystems and carbon
sequestration, to support experimental planning for large-scale MRV, and to guide decision
making on marine CDR choices.
3. New forms of iron and delivery mechanisms
Rigorous testing and comparison of new forms of iron and their potential delivery
mechanisms is needed to optimize phytoplankton growth while minimizing the financial
and carbon costs of OIF. Efficiency gains are expected to generate responses closer to those
of natural OIF events.
4. Monitoring, reporting, and verification
Advances in observational technologies and platforms are needed to support the development,
validation, and maintenance of models required for MRV of large-scale OIF deployment. In
addition to tracking carbon storage and efficiency, prioritizing eMRV will be key to developing
regulated carbon markets.
5. Governance and stakeholder engagement
Attention to social dimensions, governance, and stakeholder perceptions will be essential
from the start, with particular emphasis on expanding the diversity of groups engaged in
marine CDR across the globe. This feedback will be a critical component underlying future
decisions about whether to proceed, or not, with OIF for marine CDR.
Paramount in the plan is the need to move carefully. Our goal is to conduct these five activities in parallel
to inform decisions steering the establishment of ocean iron observatories at multiple locations in phase
II. When completed, this decadal plan will provide a rich knowledge base to guide decisions about if,
when, where, and under what conditions OIF might be responsibly implemented for marine CDR.
The consensus of our workshop and this report is that now is the time for actionable studies to begin.
Quite simply, we suggest that some form of marine CDR will be essential to slow down and reverse the
most severe consequences of our disrupted climate. OIF has the potential to be one of these climate
mitigation strategies. We have the opportunity and obligation to invest in the knowledge necessary to
ensure that we can make scientifically and ethically sound decisions for the future of our planet. |
|
|
|
|
|
| | # |
Name
(N/A = Not Available) |
Title |
Ref Date |
Date Added |
|---|
|
|
|
| Description | This is the second article in a two-part series. Read the first part here.
Climate model scenarios similar to current policies project 2°C of warming before 2050; if James Hansen is right (see Part 1) and warming sharply accelerates, it could be a decade sooner. These outcomes will be driven by the high energy imbalance, continuing high emissions, the accelerating accumulation of heat in the oceans, and decreases in short-term aerosol cooling. |
| Contents | Several years ago a group of eminent scientists proposed a “carbon law”, which said that keeping warming to 2°C required emissions to be halved every decade from 2020 onwards, including a halving between 2020 and 2030, plus some carbon drawdown. Instead, the level of greenhouse gases and coal use both hit record highs in 2023. And the largest national fossil fuel producers plan to keep on expanding production As a result, current government plans worldwide will likely result in emissions in 2050 almost as high as they are today, according to the UN Environment Programme’s 2023 Production Gap report.
Other analyses are broadly consistent:
The International Energy Agency finds that stated national policies will result in oil and gas production in 2050 as high as 2020; with coal halved.
The OECD finds that a world economy four times larger than today is projected to need 80% more energy in 2050; and without new policy action and the global energy mix in 2050 will not differ significantly from today.
The intentions of the world’s five largest fossil fuel producers are clear — and civilisation-threatening — as reported by the UN:
In China, oil production is projected to be flat to 2050, but gas will increase more than 60 percent from 2020 to 2050, while coal use will remain high till 2030 then decline sharply.
In the United States, oil production will grow and then remain at record levels to 2050, and gas is projected to continuously and significantly increase to 2050; whilst coal will drop by half.
Projections for Russia are available only to 2035, with coal and gas production projected to increase significantly, while oil remains flat.
In Saudi Arabia, oil production is projected to grow by 26 to 47 percent by 2050, with gas up 40 percent between 2019 and 2050. Together they make up half of the Saudi economy.
And in Australia, one of the world’s top two liquified natural gas and coal exporters, gas production is projected to stay above the current level for the next 15 years, with coal remaining high over the same period, above 450 million metric tons annually.
We are heading towards 3–4°C.
This outlook suggests Earth is heading towards 3°C of warming and perhaps a good deal more, because current climate models which project warming of around 2.7°C do not adequately account for all the system-level reinforcing feedbacks.
In 2021, the pre-eminent UK international affairs think-tank Chatham House said a “plausible worst-case scenario” is 3.5°C or more, which could be an underestimate if tipping points are reached sooner than the orthodox science suggests. This now seems to be the reality.
A clear majority of scientists expected warming of more than 3°C, and 82% expected to see catastrophic impacts of climate change in their lifetime, according to a 2021 survey by the journal Nature.
Questions about the size of the aerosol forcing, and the related issue of how sensitive the climate is to changes in greenhouse gases, remain an issue of scientific contention.
New climate history research published in December 2023, based on a study of the last 66 million years, concluded that global temperature may be more sensitive to CO2 levels than current models estimate. It showed that the last time CO2 levels were as high as today was around 14 million year ago, which is longer than previous estimates, and that climate sensitivity — the amount of warming resulting from a doubling of atmospheric CO2 — may be between between 5°C and 8°C, compared to the IPCC orthodoxy of 1.5–4.5°C.
The level of greenhouse gases is currently around 560 parts per million, double the pre-industrial level. Some of those gases such as methane are short-lived so this level of forcing is not written in stone, but nevertheless if Hansen et al. are right that a doubling may lead to around 4–5°C of warming, then another 30 years of high emissions means humans will have created an increasingly unliveable planet.
Has the impact of aerosols been widely understood? In what the New York Times described as “an eye-opening Nature commentary”, Geeta Persad and her colleagues wrote in late 2022 that “overall, vast emissions of aerosols since the start of the industrial age have had a profound cooling effect” and that without them “the global warming we see today would be 30 to 50 percent greater”, warning that “the impacts of aerosols on climate risk are often ignored”.
In 2018, a group of eminent scientists explored the potential — once warming had exceeded the 1.5–2°C range — for self-reinforcing positive feedbacks in major elements of the climate system to push passed a planetary threshold that would prevent temperature stabilisation, and drive the system to a “Hothouse Earth”. They warned that “we are in a climate emergency… this is an existential threat to civilisation”.
The 2023 State of the Climate Report: Entering uncharted territory warned of: “potential collapse of natural and socioeconomic systems in such a world [of 2.6°C warming] where we will face unbearable heat, frequent extreme weather events, food and fresh water shortages, rising seas, more emerging diseases, and increased social unrest and geopolitical conflict.”
Whatever the words, the understanding is widely shared that contemporary nations and societies, and likely the global social system, are heading towards collapse. “If we carry on the way we are going now, I can’t see this civilisation lasting to the end of this century”, says Professor Tim Lenton. The US Defence Secretary Lloyd Austin III calls the risks “existential”.
Opening the Innovation Zero Congress in London in May 2023, Potsdam Institute Director Prof. Johan Rockstrom described the path we are on:
“2.5°C global mean surface temperature rise is a disaster. It’s something that humanity has absolutely no evidence that we can cope with… [There] would be a 10-metre sea-level rise. There would be a collapse of all the big biomes on planet Earth – the rainforest, many of the temperate forests – abrupt thawing of permafrost, we will have complete collapse of marine biology… Over one-third of the planet around the equatorial regions will be uninhabitable because you will pass the threshold of health, which is around 30°C. It’s only in some parts of the Sahara Desert today that has that kind of average temperature.”
Chatham House’s Climate Risk Assessment 2021 concludes that by 2050 global food demand would be 50% higher, but crop yields may drop by 30%. As desertification spreads across the dry sub-tropics, and one-third of the planet experiences unprecedented heat, it is not difficult to see why they concluded that cascading climate impacts will “drive political instability and greater national insecurity, and fuel regional and international conflict”.
What is worse is the setback to climate action posed by current conflicts and military posturing in Europe, the Middle East and east Asia, which are huge political distractions from dealing with the greatest threat to humanity, and all of which have the potential to spread more widely.
To maintain military flexibility, the US insisted in 1997 that direct military carbon emissions be excluded from international carbon accounting. Those emissions, around 5 percent of the total global, are far less than the indirect emissions from conflict, as recent estimates here and here indicate.
Projections show that by 2100 the expansion of the Sahara due to desertification will embrace Israel/Palestine, as well as spreading across the Mediterranean into Spain, Italy, Greece and Turkey (see map).
The Australian Prime Minister has finally spoken out about the escalating climate threat whilst inspecting damage from the recent Queensland floods: “All of this is a reminder that the science told us that climate change would mean there would be more extreme weather events and they would be more intense. And unfortunately, we are seeing that play out with the number of events that we’re having to deal with right around Australia”.
Just so, except that in common with leaders globally, the Australian government continues to have its head stuck in the sand about the real risks climate change now represents. It refuses to release an intelligence assessment of climate-security risks, and has fumbled a domestic climate risk assessment.
As a result, the community remains ill-informed and unprepared for what is coming. |
|
|
|
| 2 |
essopenarchive.org/doi/fu... |
Exploring potential atmospheric methane removal approaches: an example research roadmap for chlorine radical enhancement |
11/8/23 |
11/8/23 |
|
|
|
| 2 |
essopenarchive.org/doi/fu... |
Exploring potential atmospheric methane removal approaches: an example research roadmap for chlorine radical enhancement |
11/8/23 |
11/8/23 |
|
| Description | The escalating climate crisis requires rapid action to reduce the concentrations of atmospheric greenhouse gases and lower global surface temperatures. Methane will play a critical role in near-term warming due to its high radiative forcing and short |
| Contents | The escalating climate crisis requires rapid action to reduce the concentrations of atmospheric greenhouse gases and lower global surface temperatures. Methane will play a critical role in near-term warming due to its high radiative forcing and short atmospheric lifetime. Methane emissions have accelerated in recent years and there is significant risk and uncertainty associated with the future growth in natural emissions. The largest natural sink of methane occurs through oxidation reactions with atmospheric hydroxyl and chlorine radicals. Enhanced atmospheric oxidation could be a potential approach to remove atmospheric methane. One method proposes the addition of iron salt aerosols (ISA) to the atmosphere, mimicking a natural process that is proposed to occur when mineral dust mixes with chloride from sea spray to form iron chlorides, which are photolyzed by sunlight to produce chlorine radicals. Under the right conditions, lofting ISA into the atmosphere could potentially reduce atmospheric methane concentrations and lower global surface temperatures. Recognizing that potential atmospheric methane removal must only be considered as an additive measure-in addition to, not replacing, crucial anthropogenic greenhouse gas emission reductions and carbon dioxide removal-roadmaps can be a valuable tool to organize and streamline interdisciplinary and multifaceted research to efficiently move towards an understanding of whether an approach may be viable and socially acceptable, or if it is nonviable and further research should be deprioritized. Here we present an example of a five-year research roadmap to explore whether ISA enhancement of the chlorine radical sink could be a viable and socially acceptable atmospheric methane removal approach. |
|
|
|
|
| Description | Climate catalyst, the main aerosol described herein, is designed to remove methane and other powerful warming pollutants from the air, adding to its cooling effect. |
| Contents | In this paper we propose that as air pollution is cleaned up, the lost cloud reflectivity could be safely replaced and further increased with benign aerosols. The effect would be to cool the world's oceans and restore more clement weather conditions. One study has suggested that carefully targeted increases of cloud reflectivity could not only restore rainfall patterns but further improve them (Ref Norwegian study).
Climate catalyst, the main aerosol described herein, is additionally designed to remove methane and other powerful warming pollutants from the air, adding to its cooling effect. However, even if only cloud reflectivity were to be increased at sufficient scale in the appropriate places, the effect would be an immediate reversal of the current warming trend. That would buy more time for the important goal of Net Zero emissions to be achieved globally. In the meantime, it would also save countless lives, infrastructure, money, ecosystems, and species from extinction. However, even if only cloud reflectivity were to be increased at sufficient scale in the appropriate places, the effect would be an immediate reversal of the current warming trend. That would buy more time for the important goal of Net Zero emissions to be achieved globally. In the meantime, it would also save countless lives, infrastructure, money, ecosystems, and species from extinction. |
|
|
|
|
Agenda
Bruce P - NOAC Website
Chris V "“ Marine CDR research projects announced.
Brian VH "“ Jim Hansen"™s Oct note "“ El Nino Fizzles, Earth Sizzles
Stephen S "“ what would doubling of aerosol do?
Clive E "“ What would satellite aerosol measurement do?
Methane PNAS conf "“ Copenhagen papers.
Chat
21:07:22 From Chris Vivian - GESAMP WG 41 : US Announcements on marine CDR:\rWhite House Forms Committee On Marine Carbon Removal - https://carbonherald.com/white-house-forms-committee-on-marine-carbon-removal/\rMarine Carbon Dioxide Removal: Potential Ways to Harness the Ocean to Mitigate Climate Change - https://www.whitehouse.gov/ostp/news-updates/2023/10/06/marine-carbon-dioxide-removal-potential-ways-to-harness-the-ocean-to-mitigate-climate-change/ \rMarine Carbon Dioxide Removal Fast Track Action Committee - https://www.noaa.gov/sites/default/files/2023-10/mCDR_FTAC_charter_2023_09_19_approved.pdf\rAnnouncing $24.3m Investment Advancing Marine Carbon Dioxide Removal Research - https://oceanacidification.noaa.gov/focus_areas/carbon-dioxide-removal/\r21:08:50 From Chris Vivian - GESAMP WG 41 : Hansen paper https://www.columbia.edu/~jeh1/mailings/2023/ElNinoFizzles.13October2023.pdf\r21:44:09 From Clive Elsworth : https://seao2-cdr.eu/\r21:47:15 From Clive Elsworth : http://www.gesamp.org/site/assets/files/1723/ocean_climate_intervention_projects_sept_2023.xlsx\r22:05:07 From baiman"™s iPhone : Yes!! Especially if aviation can emit enough aerosol to have a significant cooling impact/. Apologies I"™m with grandson so can"™t participate in person !\r22:11:20 From Hugh Hunt : https://www.ukri.org/opportunity/jet-zero-aviations-non-co2-impacts-on-the-climate/?utm_medium=email\u0026utm_source=govdelivery\r22:11:31 From Hugh Hunt : That's the JetZero link\r22:13:37 From Hugh Hunt : Maybe this one can be used for Oceans:\r\rhttps://www.ukri.org/opportunity/pushing-the-frontiers-of-environmental-research-jan-2024/\r22:21:52 From baiman"™s iPhone : Thank you Hugh\r22:31:33 From John Macdonald : Also there"™s lthe Methane Tracker 2020 |  (Click HERE to view the transcrript) (Click HERE to view the transcrript) |
|
|
|
|
| Description | Catalytic production of chlorine atoms from iron salt aerosols (ISA) has been suggested as a means of achieving atmospheric methane reduction (AMR). The feasibility of this approach its efficiency and the optimum conditions for deployment must be determined. Success is not obvious because it depends on nonlinear atmospheric free radical chain reactions; under some conditions added chlorine is known to increase methane lifetime. Here we evaluate the catalytic efficiency of atmospheric methane oxidation, initiated by the photocatalytic conversion of chloride to chlorine by iron chlorides Fe(III)Cl(3-n)n , using a OD box model. While HOx and high NOx behaviours are well known, a new regime is characterized by high ClOx conditions ypified by CH3O2 reacting with ClO rather than NO or HO2. We find that at NOx mixing ratios below 50 ppt or above 390 ppt, methane removal per iron atom is always net positive regardless of the Cl2 addition rate. However, between these NOx mixing ratios and for a chlorine production rate below 1×10^6 Cl2 /(cm3 s) the net effect is negative, increasing CH4 concentrations. The efficiencies seen in the model range from -0.26 to 2.63 CH4/Cl. |
| Contents | Catalytic production of chlorine atoms from iron salt aerosols (ISA) has been suggested as a means of achieving atmospheric methane reduction (AMR). The feasibility of this approach its efficiency and the optimum conditions for deployment must be determined. Success is not obvious because it depends on nonlinear atmospheric free radical chain reactions; under some conditions added chlorine is known to increase methane lifetime. Here we evaluate the catalytic efficiency of atmospheric methane oxidation, initiated by the photocatalytic conversion of chloride to chlorine by iron chlorides Fe(III)Cl(3-n)n , using a OD box model. While HOx and high NOx behaviours are well known, a new regime is characterized by high ClOx conditions ypified by CH3O2 reacting with ClO rather than NO or HO2. We find that at NOx mixing ratios below 50 ppt or above 390 ppt, methane removal per iron atom is always net positive regardless of the Cl2 addition rate. However, between these NOx mixing ratios and for a chlorine production rate below 1×10^6 Cl2 /(cm3 s) the net effect is negative, increasing CH4 concentrations. The efficiencies seen in the model range from -0.26 to 2.63 CH4/Cl. |
|
|
|
|
|
|